Pioneering Women
– female scientists whose skills and contributions to science were not recognized during their lifetime –
With the help of Gesine Born from the Bilderinstitut and an artificial intelligence, we are able to portray them as they deserved and as their male colleagues were lucky enough to experience.
Ada Lovelace
(1815 – 1852)
“I was born into the world a prophetess, and this conviction fills me with humility, trembling and quaking.”
picture generated by Gesine Born with AI
prompt (input you give the AI to get the picture): Wet plate photograghie of https://s.mj.run/glv9Jdv5EzQ as a 30 year old female standing confidently with one hand on her hip against a dark background. The lighting casts soft shadows across her face, creating dramatic contrast that accentuates her facial features. Her expression exudes confidence or focus, emphasizing her professional demeanor, foto from 1845 –s 50 –v 6.1 –style raw
Ada Lovelace was born Augusta Ada King Byron in London on December 10, 1815. Her father was a well-known poet, her mother a woman interested in mathematics and science. One month after Ada’s birth her parents separated, Ada never met her father. She moved with her mother to her grandparents’ country estate. Her mother, who wanted to prevent Ada from turning to literature like her father, ensured that her daughter received a sound scientific education through home tutors, including mathematics professors, and governesses.
Read more…
Ada was already exceptionally interested and inquisitive as a child. As a teenager, she was fascinated by machines. At the age of 13, she fell seriously ill and had to stay in bed for a long time. During this time, she continued to learn diligently. She dreamed of a machine that can fly. Although there were no airplanes at that time. Women were not allowed at universities at that time as well and so she wrote to scientists to learn about their research and to quench her thirst for knowledge. When she got better after a few years, she moved back with her mother to London, where she was born. Due to her parents’ aristocratic position, her mother being a baroness and her father a lord, she was introduced to court in 1833 and also had access to London’s scientific society. At a London society reception, she met the mathematician Charles Babbage and she entered into a year-long mathematical correspondence with him.
Shortly before her marriage, her diary entries state, “I believe that nothing but exact and intense occupation with subjects of a scientific nature can keep my imagination running riot and fill the void left in my mind by the hunger for experience.”
At 19, she married William King, later Earl of Lovelace, who became a member of the Royal Society on her behalf. Thus he had access to the libraries, which ladies were still barred from visiting at the time. Her husband transcribed scientific publications for her. The marriage produced three children and Ada Lovelace had then little time for her passion, mathematics, in addition to housework and family chores. Although her husband supported her, the marriage was not a happy one. Ada felt forced into the role of wife and mother, when in fact she wanted to devote most of her time to mathematics. In addition, she had limited health. “I’m one of those geniuses who limit myself to getting better.”

Her scientific contact Charles Babbage wanted to build a mathematical machine, for which he was given a lot of money, the “Differential Engine”. Ada Lovelace was one of the few who understood this scientific and technical approach among contemporaries and she became his main scientific exchange partner. The construction of this first machine devours vasted sums of money and yet was not completed.
But Charles Babbage did not let himself be stopped and wanted to build a second machine, the “Analytical Engine”. However, he was not given any more money for this. In order to promote his project and get it financed after all, he held a series of lectures that includes d-lectures in Italy. The Italian mathematician Luigi Menabrea found the lecture so inspiring that he wrote it up as a report in French. Babbage, who did not speak French, asked Ada Lovelace to translate this report into English. Because of her linguistic training, the translation was no problem for her. However, she not only translated the text, but added a total of eight notes that were three times as long as the original report. From her notes, it was clear that she saw the machine not only as an arithmetic machine, but as a computer. “The limits of arithmetic were transcended the moment the idea of using the cards was conceived, and the Analytical Engine has nothing in common with plain calculating machines. It is unique and the possibilities it suggests are most interesting.” “Cards” here refers to programming or punch cards.
The Analytical Machine “could be applied to things other than numbers, if objects could be found whose interactions could be represented by the abstract science of operations and which lent themselves to processing by the instructions and mechanisms of the device”.
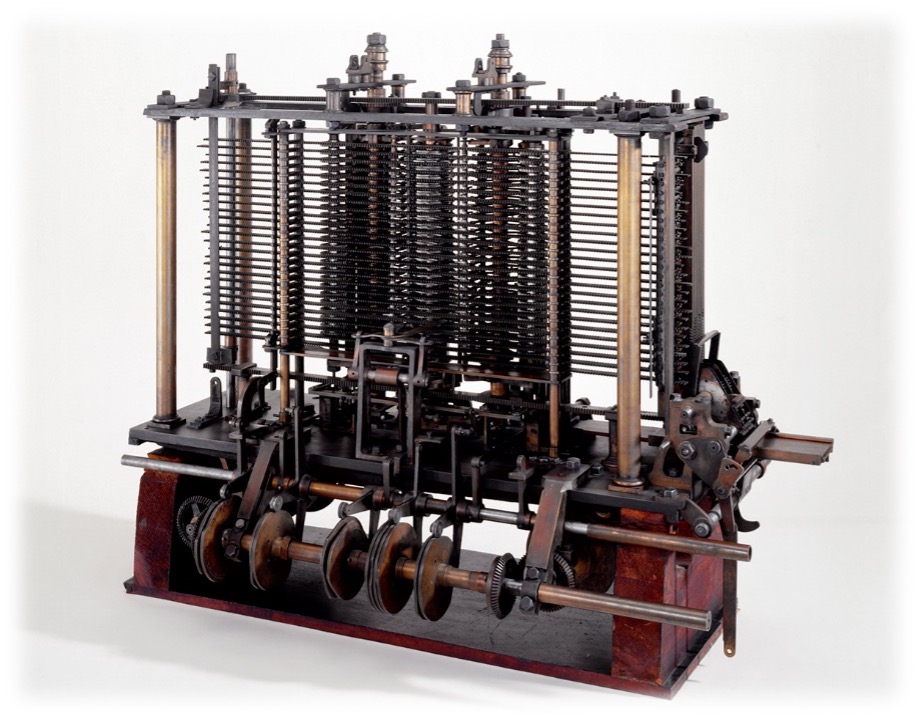
She was thinking of letters and also the programming of music, and, unlike Babbage and Menabrea, she foresaw the potential of the machine, the computer, in a visionary way. “The analytical automaton occupies a rank all to itself”.
“A tremendous new language has emerged.” She recognized that the computer would not only be able to do arithmetic in the classical sense and solve other mathematical problems, but would be able to convert any information that could be represented mathematically. The field of research what we know today as computer science. As a visionary, she was able to recognize the full potential of the machine to be developed and was thus far ahead of her time. So far that her scientific companions could not understand her ideas. It was not until 100 years later that the academic world would rediscover programming. Unfortunately, her scientific contemporaries were unable to understand the implications. To support her thesis, she programmed the first computer program, the first code. As an example she took the calculation of the Bernoulli numbers. Decisive ideas of the programming are anticipated here, like e.g. test instructions or also the innovation variables only for firmly certain purposes to use. In her program, she wrote a term with two summation signs to demonstrate two nested loops to achieve greater efficiency in the computational process. This leads to the recognition of Ada Lovelace as the world’s first female programmer. Unfortunately, she was unable to test the program in practice because the analytical machine was not built. Funding proved challenging and the necessary mechanics were not advanced enough for construction at the time.
In a table she presented the inputs and consequent outputs of the program. About her computer program she wrote: “Yes, I am very satisfied with this my first child,” which is remarkable, since she was already the mother of three children at this point. “It is an unusual baby and will grow up to be a man of first magnitude and power.”

Moreover, “Lady Lovelace’s Objections” in the research field of artificial intelligence in the field of computer science are still a subject of discussion today.
What she could not imagine at that time in this regard were self-learning machines: “The machine can do what we are able to command it to do, it can follow analysis. However, it has no ability to recognize analytical relations or truths.”

She also recognized that the functioning machine consisted of two distinguishable entities, anticipating hardware and software.
During her lifetime, her scientific achievements and foresight were hardly recognized. In addition, as the mother of three children and with her scientific and technical interests, she did not at all correspond to the prevailing image of women at the time and was therefore met with hostility. In her old age she lost further reputation, as she accumulated gambling debts while trying to find a mathematical system for horse betting. At the age of only 36, she died, presumably of cancer.
In the 1970s, the programming language “Ada” was named after Ada Lovelace. Since 1998, the Lovelace Medal has been awarded each year by the British Computer Society to outstanding computer scientists.
“I don’t think my father was as good a poet as I am going to be a good mathematician; the two are inseparable for me.”
You can download this article in German here:
Literature:
- https://www.mpg.de/frauen-in-der-forschung/ada-lovelace
- https://www.geo.de/wissen/23430-rtkl-mathematik-computer-pionierin-ada-lovelace-die-frau-die-aus-der-zukunft-kam
- GEO Epoche Nr. 105 Denker, Forscher, Pioniere 1500-1950
- https://www.welt.de/wissenschaft/article111915874/Die-Frau-die-das-erste-Computerprogramm-schrieb.html
- https://www.golem.de/news/zum-200-geburtstag-ada-lovelace-die-erste-programmiererin-1512-117919.html
- https://gi.de/persoenlichkeiten/ada-lovelace
- http://www.technolution.info/big-names-in-history/ada-lovelace
- https://www.forscherland-bw.de/mint-jobwelt/starke-frauen/ada-lovelace
If you find any errors or if you are the copyright holder of an image and do not agree to its use on this site, please contact beam@chemie.uni-halle.de .
Caroline Lucretia Herschel
(1750 – 1848)
“My father was a great admirer of astronomy and possessed some knowledge of the science. I remember him taking me out on the street one cold night to acquaint me with some of our most beautiful constellations, after we had previously observed a comet that was just visible.”
picture generated by Gesine Born with AI
prompt: wet plate photo of https://s.mj.run/SwPjeci8luc as a 30 year old female scientist standing confidently with one hand on her hip against a dark background. The lighting casts soft shadows across her face, creating dramatic contrast that accentuates her facial features. Her expression exudes confidence or focus, emphasizing her professional demeanor, foto from 1775 –s 80 –v 6.1 –style raw
Caroline Herschel was born in Hannover in 1750. Of her five siblings, there was only one other girl and four boys. Her father, a military musician, did not distinguish between girls and boys in their education and gave Caroline the same musical training as his sons. She could envision herself performing as a concert singer at an early age. Caroline went to a garrison school and learned to read and write, which was unusual for a girl at the time.
Read more…
Her mother, on the other hand, made a point of teaching her household chores. She had intended for Caroline to help the family with household chores. Caroline, however, realized early on that in addition to household chores, she also needed intellectual requirements to be happy. The Herschel family not only made music, but also philosophized and practiced astronomy.

Her brother Wilhelm Herschel, who was twelve years older, was something like Caroline’s favorite brother. He held a position as organist and concert director in Bath, England, and needed his sister as a housekeeper. At the age of 22 she followed him to England to keep house for him. But also to receive professional vocal training there and to perform as a soloist in her brother’s plays.
Soon she became the first singer, gaining a certain reputation and taking on leadership roles in the choir. In the course of this, she was offered an engagement at the Birmingham Triennial Music Festival. But she preferred to work exclusively with her brother and declined. The Herschel siblings pursued astronomy as a pastime. In 1781, her brother discovered the planet Uranus during a sky survey. This made him famous beyond the country’s borders and he was offered a position as astronomer by King George III in Slough. He gave up his musical activities and devoted himself full-time to his hobby and thus to his greatest passion. Caroline was now faced with the choice of continuing her successful musical career or accompanying her brother to Slough. She followed him and only a short time later received a lifetime appointment as his scientific assistant with an annual salary of 50 pounds.

This made her the first female astronomer who received a salary for her work. Even for many male natural scientists, this was not provided at that time. For nights on end, Caroline sat at the window and noted what her brother called out to her in terms of star positions he saw from his observation post. He looked through a very large homemade telescope.
It is said of her that had it not been for the occasional cloudy night, she would not have been able to sleep or rest at all. She also acquired algebraic knowledge and recalculated her nightly logs. She compared the records of several nights to individual stars and then calculated any changes in position. She also wrote publications for the Philosphical Transactions. In all, she collaborated on 63 publications that did not mention her name. However, Caroline not only supported her brother, she also did independent research. She discovered a considerable number of celestial bodies. She found three nebulae in 1783 and eight comets in the period 1786 – 1797. Among them was Encke’s comet, which is one of the comets with the shortest orbital periods. In 1788 her brother got married and so she was released from the domestic duties and could devote her whole day’s work to astronomy. In 1797 she had completed an index to John Flamsteed’s observations with the addition of 561 stars that were missing from his catalog. As a supplement, she had also listed the errors in the publication. Cataloging was one of her passions, and she kept a catalog of star clusters and nebulae. Today collectively referred to as deep sky objects. Caroline Herschel also helped her brother to further develop telescopes and grind lenses for reflecting telescopes, which required great manual dexterity and precision. The process involved working for 16 hours at a time.
After her brother died in 1822, she took leave of England and went back to her native Hannover. In her house she regularly received the most eminent scientists of the time, who thus expressed their esteem and favor. Her nephew John, a passionate astronomer as well, visited with family before and after his Cape of Good Hope trip, where he categorized the southern sky.
She continued her own astronomical work in Hannover and arranged her brother’s entire scientific estate. She categorized the records of their joint observations in terms of right ascension and zenith distance. The so-called “Zonenkatalog” received reduced observations of the star clusters and nebulae discovered by her brother. A cataloging that could only be accomplished through perseverance and continuous self-motivation. Considering her advanced age, this task can only be explained by absolute passion and dedication. The “Zone Catalogue of all the nebulae and clusters of Stars observed by her brother” remained unprinted, however.
In her old age she received numerous honors. In 1928 she was awarded the Gold Medal of the Royal Astronomical Society and in 1935 she was made an honorary member of the Academy. At the age of 88, Caroline Herschel became a member of the Royal Irish Academy of Sciences. At 96, she received the gold medal of the Prussian Academy of Sciences, and on her 97th birthday she was received by the Crown Prince and Crown Princess. A lunar crater was named after her with C Herschel and a minor planet bears her middle name Lucretia.
She died in January 1848, leaving behind an extensive astronomical oeuvre as well as an exciting, diverse biography in terms of how she steadily defied the ideas of the time.
You can download this article in German here:
Literature:
- https://physik.cosmos-indirekt.de/Physik-Schule/Caroline_Herschel
- https://www.google.com/doodles/caroline-herschels-266th-birthday
- https://www.mpg.de/frauen-in-der-forschung/caroline-herschel
- https://www.fembio.org/biographie.php/frau/biographie/caroline-herschel/
- https://www.deutsche-biographie.de/sfz30292.html
- https://astrokramkiste.de/herschel-caroline
- https://www.deutschlandfunk.de/grosse-astronomin-vor-270-jahren-geboren-caroline-herschel-100.html
- https://www.belladonna-bremen.de/frau-des-monats-november-2021-caroline-herschel/
- https://www.welt.de/geschichte/kopf-des-tages/article232849263/Caroline-Herschel-Die-erste-bezahlte-Astronomin-der-Welt.html
If you find any errors or if you are the copyright holder of an image and do not agree to its use on this site, please contact beam@chemie.uni-halle.de .
Clara Immerwahr
(1870 – 1915)
“I have only ever known the joy of life in fleeting moments, and I may well say that each of them has outweighed the years of hardship”
picture generated by Gesine Born with AI
prompt: Daguerreotypie fotografie from 1900 of 30 year old female scientist https://s.mj.run/_86DEV7kZLQ standing proud and confident with crossed arms in front of a dark backround –v 6.1 –style raw
Clara Immerwahr was born on June 21, 1870. She had two older sisters and an older brother. Her father was a chemist and later a farmer who experimented with artificial fertilizers in his own laboratory. Clara, like her two sisters, received private lessons in the summer at the family’s country estate. In the winter, the family moved to Breslau and the sisters went to a high school for girls. The principal of this school recognized Clara’s talent and arranged for Clara to receive additional private lessons from the school’s chemistry teacher.
Read more…
She also gave her the reference book “Conversations on Chemistry.” After graduating from school, Clara Immerwahr attended the teacher training seminar to obtain the highest possible degree for women at that time. Afterwards it cost her a lot of energy, but in the winter semester of 1886 she was able to attend the lecture “Experimental Physics” at the University of Breslau as a guest student. She was not deterred by the resistance of the professors, one of whom made it clear that he did not think much of “intellectual Amazons”.
She wanted more and so she gained access to the laboratories to experiment. The chemist Richard Abegg recognized her talent. She assisted him in experimenting and he became her scientific mentor. In 1897, she obtained special permission to take the Abitur as an external student at a boys’ high school. After passing the Abitur, she was able to enroll as a full student. After completing her studies, she began working on her doctorate under Richard Abegg. In March 1900, she wrote of herself with conviction: “I ask Mr. Professor not to be angry with me if, on the basis of my experiences, I warn Mr. Professor on the basis of my experience to rely on Gauss’ measurements” to Abegg. She was awarded a magna cum laude doctorate in physical chemistry in the same year. Her dissertation was entitled
“Contributions to the Solubility Determination of Poorly Soluble Salts of Mercury, Copper, Lead, Cadmium, and Zinc.” Here she developed a measuring method that makes it possible to detect the changes in the electrical energy of metal ions in salts. Today, this solubility determination of heavy metals still plays an important role in electric motors, batteries and for measuring the pollution of the oceans by chemicals.

The “Provinzial-Zeitung” wrote about her disputation on December 22: “Our first female doctor. Saturday noon 12 o’clock sine tempore the doctorate of Miss Immerwahr took place in the Aula Leopoldina of our Alma Mater.” She defended herself “valiantly and bravely as a man” and presented herself “exceedingly suave and quick-witted.” The dean spoke of her as a shining example and clarified that she achieved the desired goal under the most difficult conditions. He further emphasized that everyone, regardless of gender, denomination, race, and nationality, is welcome to science.
Clara Immerwahr married Fritz Haber in 1901, although she had rejected his marriage proposal in previous years. She also asked for time to think about the second proposal. In retrospect, she wrote: “It was always my view of life that it was only worth living if one had developed all one’s abilities to the fullest and had lived through as much as possible… And so it was with this impulse that I finally decided to marry, that otherwise a decisive page in the book of my life and a chord of my soul would remain fallow.” She entered into the marriage with the thought of a union like that of the “Curies.” Fritz Haber promised her, that she could continue research at his side. It looked like it might work: “I now work every afternoon at the Institute, reading and making drawings to go with it,” she wrote to her former mentor Richard Abegg.
But already after the birth of their son Hermann, the complexity of this claim became apparent. In the social norms of the time, the possibility of reconciling career and family was not provided for women. Clara felt deprived of her own scientific possibilities, goals, challenges and visions in the role of motherhood, housewife and representative professor’s wife. These applicable requirements did not fit with her parenting style and beliefs.
“But I am unlikely to get to work in the laboratory again, for my days are amply filled with work. Maybe again later, when we are millionaires and can keep a servants’ staff. But even in my thoughts I can’t completely do without it.”
In the winter semester of 1905/1906, she offered a series of lectures on “Chemistry in the Kitchen and Home” at the Volksbildungsverein in Karlsruhe. “I give lectures to about 100 female listeners: The ladies are enthusiastic.”
In 1906, Fritz Haber received a full professorship in Karlsruhe; two years later, he achieved a breakthrough with ammonia synthesis.

In 1909, she expressed her feelings very characteristically in a letter to Richard Abegg: “What Fritz has gained in these eight years, that – and even more – I have lost, and what is left of me fills me myself with the deepest dissatisfaction. If I wanted to sacrifice even more of the little right to life that has remained for me here in Karlsruhe, I would let Fritz dry up into the most one-sided, albeit most significant researcher imaginable. And I ask myself whether superior intelligence is enough to make one person more valuable than another, and whether many things about me that go to hell because they didn’t get to the right man aren’t worth more than the most important theory of electron theory?”
About marriage she wrte: “The upswing I had from it (marriage), however, has been very short. … Thus the main part (of the discontent) is to be written on Fritz’s crushing opinion for his own person in the house and in the marriage, beside which simply every nature which does not assert itself even more ruthlessly at his expense perishes. And that is the case with me.”
In 1910 the family moved to Berlin-Dahlem because Fritz Haber became director of the Kaiser Wilhelm Institute for Physical Chemistry and Electrochemistry.
At the beginning of the First World War, Fritz Haber enlisted as a war volunteer. Somewhat later, he headed the “Central Office for Questions of Chemistry”. He was instrumental in driving forward the
development of poison gases. Clara saw this as a “perversion of science” and called his efforts “a sign of barbarism, corrupting that discipline which was supposed to bring new insights to life.” When she accompanied her husband to a military training ground near Cologne, she made a speech full of passion that went unheard. “If you were really a happy person, you couldn’t do this.” After the first poison gas attack in April 1915, Fritz Haber was promoted and celebrated this on May 1 at his villa in Dahlem. In the early morning of May 2, Clara Immerwahr shot herself with her husband’s service weapon. A surviving quote from Fritz Haber on this: “She could not bear life and left the field early in the morning on the day I had to go to Galicia again. I have no time to look right and left, to think and to sink into my feelings. It is a real relief for me to be in front where the bullets are hitting. But then one sits again with the general command and hears in the heart the words, which the poor woman spoke then and then and sees in the vision of the tension her head emerge and suffers”. Fritz Haber went back to the front the same day, which he would not have had to do under the circumstances, leaving his son behind in Berlin.
Clara Immerwahr did not have an easy time realizing herself and her goals during her lifetime. After her death, she was declared insane and her existence as a scientist and wife of Nobel Prize winner Fritz Haber was ignored. The reasons for her death were swept under the rug. Photos of her and her farewell letters were destroyed. The source material for Clara Immerwahr was therefore sparse. In the archives of the Max Planck Society, the successor institution of the Kaiser Wilhelm Society, there are three thin DIN A4 folders; in contrast to those of the wives of the other directors, who are well documented.
Although Clara Immerwahr had taken her husband’s surname after marriage, she is listed in the literature and thus also in this text under her birth name “Immerwahr”.
Today, two Clara Immerwahr Awards at the Technical University of Berlin and the Technical University of Kaiserslautern commemorate her.
You can download this article in German here:
Literature:
- Chemikerinnen – es gab und es gibt sie, Gesellschaft Deutscher Chemiker, 2003
- Clara Immerwahr (1870-1915): Die erste deutsche Doktorin der Chemie https://www.gdcf.de/publikationen/biographien-von-chemikerinnen/clara-immerwahr.html
- Clara Immerwahr – Späte Ikone für den Frieden von Rainer Volk, https://www.swr.de/swr2/wissen/clara-immerwahr-swr2-wissen-2020-12-29-100.html
- Selbstmord aus Protest von Gerit Leitner https://www.deutschlandfunk.de/clara-immerwahr-selbstmord-aus-protest-gegen-chemische.871.de.html?dram:article_id=318676
- Das Chemie-Unglück von Erwin Starke https://www.tagesspiegel.de/gesellschaft/der-tod-von-clara-immerwahr-das-chemie-unglueck/11620096.html
If you find any errors or if you are the copyright holder of an image and do not agree to its use on this site, please contact beam@chemie.uni-halle.de .
Emmy Noether
(1882 – 1935)
“I always went my own way in teaching and scientific research.“
picture generated by Gesine Born with AI
prompt: vintage wet plate portrait of 50 year old female scientist https://s.mj.run/-OVKarJI560 waering a white shirt with a black bow tie, sitting in front of a dark curtain, Foto from 1910 –s 250 –v 6.1
Amalie Emmy Noether was born in Erlangen in 1882, the eldest child of four siblings. She had three brothers. The family lived in Erlangen, where she went to school. From 1889 to 1897 she attended the Höhere Töchterschule. This was followed by three years of private preparation for the language examination. She passed the exam in 1900 and was able to work as a teacher of French and English. However, she was only allowed to teach women. Because of her Jewish background, she had to contend with the additional restriction that most schools were Protestant or Catholic and therefore did not want to employ her.
Read more…
Since women were not allowed to enroll in universities at that time, she attended mathematics lectures as a guest student at the University of Erlangen for two years. Her father was a professor of algebra at this university.

In addition, her student life included attending lectures in Romance languages and literature and history. In 1903 she externally passed the Abitur at the Realgymnasium in Nürnberg and decided to go to Göttingen. At that time, the university town of Göttingen was considered the Mecca of German mathematics. However, even here only a guest student status was possible.
Starting in the winter semester, women were allowed to enroll at Bavarian universities, and so Emmy Noether returned to Erlangen to continue her studies and earn her doctorate. In 1907 she wrote her doctoral thesis on the problems of invariant theory with Paul Gordan. Her dissertation was entitled “On the Formation of the Form System of the Ternary Biquadratic Form.” A year later this manuscript was published in the “Mathematische Annalen“. Later, she said about her doctoral thesis “calculating” and “formula tangle”. In the period after her doctorate, as the only woman among 47 mathematics students, she held lectures as a substitute for her sick father and also two of her doctoral supervisor’s successors. However, she had no regular employment or university position.
During this period she also produced numerous publications that made her known as a mathematician. Thus, in 1908 she became a member of the Circolo Mathematico Palermo. After she also became a member of the German Mathematical Association in 1909, she was the first woman to give a speech at its annual meeting.
The mathematicians David Hilbert and Felix Klein brought her back to Göttingen in 1915. They hoped she would help them understand the theory of relativity. Emmy Noether’s application for habilitation, which she first submitted in 1915, was rejected despite Hilbert’s and Klein’s influence. The mathematical-scientific department approved the application, but the historical-philological department of the philosophical faculty rejected it. As a result, it was still not allowed to hold lectures in her own name.

The lectures are announced under the name of the professor and the addition “with the support of Frl. Dr. Noether”. After the end of the First World War there was finally the possibility for women to habilitate. Mrs. Noether submitted a manuscript on “Invariant Variational Problems.” The paper was about the connection between conservation laws and symmetries.
This exposition made her famous among physicists. Even today this is the basis in classical mechanics and the field theories in electrodynamics and gravitation. This publication and a previous publication hardly mentioned today helped Albert Einstein, David Hilbert and Felix Klein to penetrate the connections, on which the covariant equations are based, in the general relativity theory. Einstein wrote to Hilbert on 24.05.1918: “Yesterday I received from Fr. Nöther [sic] a very interesting paper on invariant formation. It impresses me that one can overlook these things from such a general point of view. It would have done the Göttingen field grays no harm if they had been sent to Miss Nöther’s school. She seems to know her trade well.”
From Albert Einstein’s letter to Felix Klein, December 27, 1918: “On receiving the new work of Frl. Noether, I again feel it a great injustice that she is being deprived of the venia legendi. I would be very much in favor of taking an energetic step with the Ministry”.

The habilitation and thus the authorization to teach was finally granted to her after three attempts with an exemption. She had made her first attempt at this in 1915 and then, after being asked to do so, a second attempt in June 1917. The third successful attempt began in 1919. On June 4, 1919, she gave a lecture on the questions of module theory and in the following semester the lectures she gave were now finally announced under her name. Emmy Noether was very productive and published a wealth of papers. In 1921 she gained international renown in the field of algebra with her publication “Ideal Theory in Ring Domains.” The following year she became an associate professor. This was a position outside the civil service with little income. Four years later she published the manuscript “Abstract structure of ideal theory in algebraic number and function fields.” In1928 and 1929 she was in Moscow for a visiting professorship and in 1930 in Frankfurt am Main. In 1929 she published her manuscript “Hypercomplex quantities and representation theory.” At the International Congress of Mathematicians in Zurich in 1932, she gave the keynote lecture! In addition, she was awarded the Ackermann-Teubner Memorial Prize in 1932.
Already in 1933 she published another much acclaimed paper, “Noncommutative Algebras.” Emmy Noether left her mark on a large circle of students and colleagues. Many came to Göttingen from all over the world just because of her, and a large number of very talented mathematicians contributed as former students of Noether to spread and develop her ideas. She established the Noether School in Göttingen in which she created a thinking space of clear thinking. Her school was demanding; you had to be better than everyone else. But by her own personality and the quality of determination as well as the benevolent kindness of her social competence she established a fruitful exchange. The members of the Noether School were not only students but also professors.

However, like many of the Jewish scientists, Emmy Noether was dismissed in 1933 and emigrated to the USA. Through the support of the head of the college’s mathematics department Anna Wheeler and colleague Hermann Weyl, she received a visiting professorship at Bryn Mawr Women’s College in Pennsylvania.
The college is located 50 miles from the university town of Princeton, where she additionally lectured at the Institute for Advanced Study, founded in 1930.
Emmy Noether died unexpectedly in 1935 as a result of surgery.
Today, Noether’s rings and Noether’s theorem are named after her. “More importantly, she was a good person through and through, free from all egotism, free from all vanity, free from posturing, and she always helped everyone where she could. Her lectures were not beautifully polished. She recited what she had just thought up and she tried to improve the presentation even during the lecture. It went like this: even before she finished a sentence, she would very quickly come up with a better formulation.”

‘The proof is now abstractly formulated and made transparent.’ For this was for her the meaning of modern abstract algebra, that all special calculations ‘with matrices, etc., were avoided, that one abstracted from all unessential features of the special problem, and that through this abstraction the essential became visible, the terms were placed at the top, and the whole proof became transparent. “1
You can download this article in German here:
Literature:
- https://www.dfg.de/foerderung/programme/einzelfoerderung/emmy_noether/person/index.html
- https://www.dhm.de/lemo/biografie/biografie-emmy-noether.html
- https://www.mathematik.de/des-monats/773-emmy-noether
- http://www.mathe.tu-freiberg.de/~hebisch/Praktikum/noether.html
- https://www.erlangen.info/noether/
- https://www.lernhelfer.de/schuelerlexikon/mathematik-abitur/artikel/emmy-noether
- https://www.fembio.org/biographie.php/frau/biographie/emmy-noether/
- 1Bartel van der Waerden am 26. Januar 1979 im Hörsaal der Chemie in Heidelberg bei einem Vortrag über “Meine Göttinger Lehrjahre”. Van der Waerden sprach frei, der Vortrag wurde auf Tonband aufgenommen, transkribiert und von Peter Roquette 1997 veröffentlicht.
If you find any errors or if you are the copyright holder of an image and do not agree to its use on this site, please contact beam@chemie.uni-halle.de .
Frieda Nugel
(1884 – 1966)
“Don’t elect lame ducks, but women who have an understanding and interest in the work of public life…”
picture generated by Gesine Born with AI
prompt: female scientist standing confidently proud and serious with crossed arms in front of a dark background –cref https://s.mj.run/jAF_HTWjZ0U –cw 100 –v 6.1 –s 150 –style raw
Frieda Nugel was born in Cottbus on June 18, 1884. She had three older and two younger siblings. Her father initially taught her at home. Frieda Nugel then attended the Higher Girls’ School in Cottbus from 1901 – 1906. Attendance at this school was associated with the teacher training seminar. However, she did not take the examination to become a teacher for middle and high schools for girls at the school in Cottbus, but at the Royal Elisabeth School in Berlin.
Read more…
She then worked as a home teacher for one and a half years. At the same time, she took private lessons with a mathematics professor to prepare for the school-leaving examination. At that time, this examination could also only be taken in Berlin.

In the fall of 1907, Frieda Nugel passed the examination at the Luisenstädtische Oberschule in Berlin. After graduating from high school, she began to study mathematics, physics and German in Berlin. After three semesters, she transferred to the University of Munich for one semester. From the winter semester 1909 -1912 she studied at the University of Halle-Wittenberg. The year 1912 was an eventful one for her.
As early as February 1912, she received her doctorate with a thesis on “The Helical Lines: A Monographic Presentation.” Her oral doctoral examination in mathematics, physics, and philosophy took place over two days on February 27-28, 1912. In May she became engaged and on July 9, after the dissertation was printed, she received her doctoral certificate. Less than three weeks later, on July 27, she passed the state examination in mathematics, physics and German. Employment followed immediately in July in Cottbus at the Higher Girls’ School, which she herself had once attended, as a senior teacher. From this year on, she was also a member of the German Mathematicians’ Association, which she remains until 1929.

In 1914 she married her fiancé Louis Hahn and went with him to Westphalia, where she worked as a teacher until the end of the year. Their first of four children was born in March 1915. At the end of the year, the family moved to Emden. Here in Emden, Frieda Nugel worked as a teacher from Easter to December in 1916. From Easter of the following year she was rehired and worked until the end of 1918 as a so-called “war substitute” at the Kaiser-Friedrich-Oberrealschule. Which is remarkable, considering that in March 1917 her second child was born. In addition, she gave private lessons in mathematics, German and physics from 1918 until 1928. During this time period, she also published on the topic of women’s rights and advocated for improved educational opportunities for women and girls. She encouraged women to go to the polls and vote. “Don’t elect lame ducks, but women who have an understanding and interest in the work of public life…”
She denounced the unquestioned sole destiny of women as mothers and homemakers. She was concerned with showing possibilities for self-fulfillment in addition to hearth and family.
In 1920, after a daughter and a son, her second son was born. Her youngest daughter was born two years later in 1922. In addition to her family duties and the private lessons she gave, she also supported her husband in his journalistic activities. He was a historian and Germanist and worked as managing director in the “family business” of the Ostfriesische Zeitung. During the world economic crisis, however, the newspaper had to cease publication. Her husband then published mainly on a voluntary basis and Frieda Nugel had to earn the family living virtually alone. In order to improve her financial position, she worked from 1927 – 1928 as a real teacher in a salaried relationship with, however, only four hours of weekly teaching. In order to obtain a better professional position, she qualified externally in Berlin as a scientifically educated teacher and then became a study accessor on October 1. Two years later she became a lecturer, but with 10% lower earnings than her male colleagues.

She at least had the advantage that this position was a permanent one and, as a married woman and mother of four children, she could not be transferred. She taught physics, mathematics and German, and in 1934/35 she also taught geography. In 1945, at the age of 61, she retired from teaching. Seven years later her husband died.
In 1955 she moved to Bad Godesberg to be with her youngest daughter and to support her in the work and care of her three grandchildren. Frieda Nugel was awarded the “golden doctorate diploma” in 1962. Her dissertation of 1912 was classified by a professor in Tübingen as “particularly valuable in terms of mathematical history” and professors of the Humboldt University applied for the award from the University of Halle. The certificate was then presented to Frieda Nugel by Prof. Beck of the University of Bonn at her home. Frieda Nugel died at home in Bad Godesberg on November 6.
You can download this article in German here:
Literature:
- https://disk.mathematik.uni-halle.de/history/nugel/index.html
- Die Frau in der Gemeindeverwaltung
Frau und Welt: Deutsche Allgemeine Zeitung (1921) Nr.38 vom 24. September.
If you find any errors or if you are the copyright holder of an image and do not agree to its use on this site, please contact beam@chemie.uni-halle.de .
Liselotte Feikes
(1923 – 2008)
“Start and do what you like to do, because what you like to do, you succeed with”
picture generated by Gesine Born with AI
prompt: Hasselblad black and white Portrait, 80mm from 1975 of 50 year old female scientist standing confidently, proud and serious in front of a dark background, wearing a suit –cref https://s.mj.run/Q2L2fCy2ghQ –cw 0 –v 6.1 –s 20 –style raw
Liselotte Feikes was born in Viersen in 1923. In the middle of the Second World War, she graduated from high school in 1942. She received her diploma on March 25. Not even a week later on April 1, labor service already began, followed by military service.
Read more…
Her mother had already contacted relatives in nearby university towns when the war was on the horizon, asking them to arrange something. Mrs. Feikes quoted her mother as saying, “You can do what you want, but what you start, you finish.” With the help of a letter from relatives, she was able to enroll in the chemistry program in Halle for the summer semester of 1943. She studied in Halle for three semesters, and the following winter semester, 1944/1945, she was again required to do military service. However, “the head of the institute, Professor Karl Ziegler, … knew how to prevent that some chemistry students have to do it.” Thus, in October 1944, she became an assistant to Mrs. Becke-Goehring and experienced the end of the war as an employee of the Chemical Institute. In the summer of 1945, she returned to her birthplace of Viersen and sought employment, so from there she contacted Mrs. Becke-Goehring, who was now working as a lecturer in Heidelberg. At that time one still telegraphed and some time later a telegram reached her with the question “whether I would like to start with her as a laboratory assistant.” Mrs. Feikes immediately agreed, telegraphing back “yes.” Based on her “papers,” she was admitted to study in Heidelberg quite quickly and was able to continue her studies. After graduation, she also completed her doctorate under Mrs. Becke-Goehring, who had, after all, brought her to Heidelberg. The dissertation is dated July 1, 1953, and is entitled “On the conversion between polythionate ions and sulfur water vapor.” It so happened that the day after the examination ceremony she was called to the director of the Institute of Chemistry Prof. Freudenberg. “He started the conversation: `My brothers in Weinheim…`. Those were the two entrepreneurs. In Weinheim, eight days later, the conversation started: `My brother in Heidelberg.`. That was really all,” she later said in an interview about her career start at Freudenberg. Her Ph.D. in analytical chemistry led to her being hired because they were looking for “someone specifically for analytical tasks.” Her first field of activity was the inventory of wastewater at the Freudenberg tannery. Three years later in 1956, the chemist in charge of the tannery’s testing laboratory died and “so I was able to step in.” Her area of responsibility now expanded to include environmental protection. The company built its own wastewater treatment plants, in the process development and plant construction of which it played a major role. The company’s own wastewater treatment plants were built all over the world and she advised the international branches of the Freudenberg Group. Grenoble, Rio and Mexico City benefited from her knowledge. She was able to work internationally for years because she understood something about both leather production and wastewater. “The key thing has always been the combination between environmental protection and leather production…. Today, people think more in terms of divisions and have blinkers.”
At Freudenberg, many similar issues arose regarding plant engineering and environmental protection. It became necessary to have a contact person for internal issues within the company and a person to represent the company’s interests externally. So in 1972, Ms. Feikes became the Group’s Environmental Protection Officer.
When asked what special experiences she had as a woman in industry, Ms. Feikes replied, “As a woman, you have to prove more often that you can answer questions. I would never have been able to do my job the way I did if I had obligations to anyone, be it spouses, be it parents or children. You can’t do certain things unless you’re independent.”
Ms. Feikes was committed to the environment combined with leather technology. A 1974 presentation at the annual conference of the Association of German Auditing Engineers (VDRI) was titled “Disposal of Industrial Waste Materials. Industrial Viewpoints” and is available on the Internet today. In 1979, she received the annual award of the Association for Tannery Chemistry and Technology as an award for her work on environmental problems in the leather industry. She wrote a book on the “Ecological Problems of the Leather Industry,” which was published in 1983, and a year later became an honorary member of the Association of Austrian Leather Technicians. She received the highest award, the Federal Cross of Merit on Ribbon, in 1985 for her commitment to the field of environmental problems. In 1986 she gave the John Arthur Wilson Memorial Lecture. A lecture on theoretical and applied leather technology initiated by the industry.
You can download this article in German here:
Literature:
- AKCC Arbeitskreis Chancengleichheit in der Chemie der Gesellschaft Deutscher Chemiker, 2003
- https://onlinelibrary.wiley.com/doi/abs/10.1002/nadc.20020500532
- VDRI Jahrbuch 1974
- VDRI Jahrbuch 1976
If you find any errors or if you are the copyright holder of an image and do not agree to its use on this site, please contact beam@chemie.uni-halle.de .
Ida Tacke-Noddack
(1896 – 1978)
“It is beautiful to research and you can’t get away from it at all, and I don’t want to stop researching either”
picture generated by Gesine Born with AI
prompt: Vintage foto from 1925 of 30 year old female scientist standing confidently, proud and serious in front of a dark background –cref https://s.mj.run/pOkfaG0RdjI –cw 0 –v 6.1 –s 150 –style raw
Ida Tacke was born on February 25, 1896. She attended the secondary school for girls in Wesel (NRW). In 1908 women were admitted to the university, so she graduated from high school. There was a girls’ high school nearby, in Aachen, but it did not yet have examination authorization. It was therefore also necessary for her to take the Abitur examination externally. She began her chemistry studies in 1915 at the Technical University in Berlin.
Read more…
Her father supported her in her endeavors. Since he was a paint manufacturer, he possibly hoped that Ida could work in his company. Although women were admitted to the university, there were still lectures that were not open to them. So, without further ado, Ida Tacke went to the lecture dressed as a man. She was aware of the carrying power of women’s studies and therefore joined the “Association of Studying Women.”
She graduated in chemistry in 1919. In the same year, she received the 1st prize of the Department of Metallurgy of the Technical University for another scientific work. With her dissertation entitled “Die Anhydride höherer aliphatischer Fettsäuren” (The Anhydrides of Higher Aliphatic Fatty Acids), she received her Ph.D. in 1921. After two years in business at AEG, she took the opportunity to return to research and began conducting research at the Physikalisch-Technische Reichsanstalt in Berlin. She used X-ray spectrography to detect elements in the microgram range. It was here that she met her future husband, Walter Noddack. The two researchers devoted themselves to the search for the missing elements in the periodic table, which Mendeljew had predicted, with the numbers 43 and 75. Due to the circumstances of the time, she remained in the background and remained her husband’s scientific collaborator throughout her life. The element 75 was discovered spectroscopically by Ida in 1923, and they called the element rhenium as a reminder of their Rhenish origin. The first quantities of the element were enriched from columbite. This was physically strenuous work, which additionally involved travel to the respective mining areas. This was because the elements to be found have their deposits in the rocks of the earth’s crust. In order to enrich a valuable gram of this element, they worked up a fabulous 660kg of molybdenite from Norway in 1927. The confirmation of rhenium was not long in coming, whereas element 43, which they called masurium in reference to Walter’s origin and also discovered in this period, was doubted. They could not detect the element by X-ray spectroscopy. It is now known to be radioactive and to decay very rapidly. In 1937 this was finally assumed to be certain and it was called technetium. Therefore, hardly anyone knows the name masurium today.

In 1926, Ida Tacke and Walter Noddack got married. In the same year, Ida Tacke-Noddack gave her first major lecture to ~900 chemists. This was still a sensational event at the time. “Today, for the first time, a girl spoke – and she even did it well” said a contemporary voice. A strong piece!
In a 1934 issue of “Angewandte Chemie” she expressed the following conjecture, anticipating nuclear fission. “It would be conceivable that when heavy nuclei are bombarded with neutrons, these nuclei decay into several larger fragments which are isotopes of known elements but not neighbors of the irradiated elements.” At that time this was contradictory to the usual assumptions and even more so by a woman, no attention was paid to this idea. Otto Hahn is quoted on this with “Your assumption of the bursting of the atomic nucleus is absurd after all.” Five years later, nuclear fission was practically demonstrated by Otto Hahn and Fritz Straßmann. Lise Meitner also had her share in this.
Ida Tacke-Noddack’s share was awarded late, in 1966, by Otto Hahn’s saying “and Ida was right after all”.
In 1931, Ida Tacke-Noddack was the first and until today (2021) the only woman to receive, together with her husband, the Liebig Medal of the Society of German Chemists. In 1937 she became a member of the Leopoldina. The couple’s career took them via Freiburg im Breisgau and Strasbourg to Bamberg. From 1945 onwards, they set up the Research Institute for Geochemistry here. At that time it was, together with the one in Moscow, only one of two of its kind in Europe.

Ida continued to devote her research to the discovery of elements. The Noddack couple started from the ubiquitous occurrence of the elements. They thus established the chemistry of trace elements. By assuming the “ubiquity of elements”, they conducted studies of marine animals and meteorite rocks, unusual for that time.
Walter Noddack died unexpectedly in 1960, and the obituary for her husband says: “…that almost from the beginning of his scientific career until the end of his life he could enjoy the untiring and self-sacrificing cooperation of his wife Ida.”
But she continued to do research in biochemistry without him and also published papers in the field of photochemistry and geochemistry. Examples of this include the visual pigments of the human eye, the carbon cycle, and how kidney stones can be dissolved. In addition, Ida Tacke-Noddack is considered a founder of cosmochemistry through her investigations of meteorite rocks. Ida Tacke-Noddack’s scientific productivity is reflected in about 60 published papers. In 1966 she was awarded the Grand Cross of Merit of the Federal Republic of Germany. Ida Tacke-Nodack was nominated for the Nobel Prize four times between 1933 and 1937. In 1933 by Walther Nernst and Karl Wagner, in 1935 by Wolf Müller and in 1937 by Anton Skrabal. She did not receive it. Her husband was nominated nine times in the same period, but did not receive the prize either. Unfortunately, the certificate or diploma thesis, or dissertation, has not been preserved at the TU Berlin, as the records of the Technische Hochschule Berlin were almost completely destroyed in an air raid in 1943. Likewise, the university library has suffered heavy losses, so the documents are no longer available here either.
You can download this article in German here:
Literature:
- Ida Noddack: „Über das Element 93“, in Angewandte Chemie 1934, 47. Jahrg. Nr. 37, S. 653 – 655.
- Ida Noddack: „Das periodische System der Elemente und seine Lücken“, Angewandte Chemie 1934, 47. Jahrg. Nr. 20, S. 301
- www.frauenruhgeschichte.de
- www.chemie-schule.de
- www.chemie.de/lexikon/Ida_Noddack-Tacke.html
- www.fembio.org/biographi.php/frau/biographie/ida-noddack
If you find any errors or if you are the copyright holder of an image and do not agree to its use on this site, please contact beam@chemie.uni-halle.de
Gerty Cori
(1896 – 1957)
“It seems to me that men are short-sighted who think that by suppressing science other creative forces will be released.”
picture generated by Gesine Born with AI
prompt: vintage foto from 1950 of elderly looking 40 year old female scientist https://s.mj.run/OYkv5jRaO0U with messy hair and a black suit, looking straight and confident in the camera, standing in front of a black backround with crossed arms –s 250 –v 6.1
Gertrude Theresa Radnitz was born in Prague on August 15, 1896. She was the eldest of three sisters. She was educated at home until she was enrolled in a girls’ school in 1906. She finished this school in 1912 and then prepared for the baccalaureate to gain entrance to the university, which she took externally at the Tetschen Realgymnasium in 1914. She then began her medical studies at the German University in Prague.
Read more…
An uncle, who was himself a pediatrician, had encouraged her to study medicine. During her studies, she met Carl Ferdinand Cori. Commonalities such as hiking and skiing and similar scientific interests led them to become a couple. They married in 1920, the same year she finished her studies. Gertrude Radnitz took her husband’s last name.
They moved to Vienna after graduation and Gertrude Cori worked for two years as an assistant physician in a children’s hospital. At that time she was also researching the connection between the regulation of body temperature and the thyroid gland. Due to the tense situation after World War I, the Coris emigrated to Buffalo in the United States in 1922 and became American citizens in 1928. Gertrude Cori, however, followed her husband to Buffalo six months later because she could not get a position before then. Both had a greater interest in research than in the medical-clinical side of the medical profession. The Cori couple always did research together, although this initially led to difficulties in finding employment for Ferdinand Cori. Some universities suggested to Ferdinand Cori that he should no longer work with his wife, as she would slow down his scientific career. This even led to the point that he was once offered a professorship only on the condition that he would no longer work with his wife.

Cornell and Toronto Universities refused to hire Gertrude Cori. The University of Rochester warned Gertrude Cori that she would ruin her husband’s career by collaborating with her. Gertrude Cori had to endure such discrimination for a very long time in her life. Her husband then got a position at the government institute for cancer research.
She was only given a position as a pathology assistant. She was told that if she left the lab to work with her husband, she would be fired. The Coris did not relent and eventually they were allowed to work together in one institute. Although Gertrude Cori had the same qualifications as her husband, she was given only an assistant position and one-tenth of her husband’s salary.
Beginning in 1931, her husband headed the pharmacology department at St. Louis University. Gertrude worked as his research assistant in biochemistry with no salary! They always did research together, but only her husband made a career.
The couple then moved into biochemistry. They did research on glucose metabolism. In 1936, they isolated glucose-1-phosphate. In the course of this, they also discovered phosphorylase. Also following this, they were able to demonstrate the in vitro synthesis of glycogen and starch. They were the first scientists to succeed in synthesizing a physiological macromolecule in vitro. In further experiments they were able to crystallize phosphorylase and other enzymes. The couple had offspring in 1936, when their son was born.

Later, they were also able to elucidate the metabolism of lactate and glucose between skeletal muscle and the liver. During muscle work, glycogen is broken down into glucose for energy production, producing lactate, which is then transported to the liver. Here, the lactate is synthesized back into glucose using energy and stored as glycogen.
The synthesized glucose from the liver is also transported back to skeletal muscle as needed. The glycogen reserves of the liver and skeletal muscle are therefore linked via this cycle. This linkage of muscular glycolysis (glucose breakdown) and hepatic gluconeogenesis (glycogen buildup in the liver) is now called the Cori cycle. Muscle cells shift their metabolic load to the liver via this cycle. The Cori cycle was published in 1940. In 1947, they jointly received the Nobel Prize in Medicine or Physiology for it: “for the discovery of the enzymes that convert glycogen to sugar and back to glycogen.”

In 1947, Gertrude Cori also finally received a full professorship in biochemistry. For 16 years she had only been able to work as an assistant. She did not become a professor until her husband was appointed head of an institute. She accepted the professorship even though she developed myelofibrosis that year. She continued to work undeterred until she died at the age of 61 on October 26, 1957.
“I believe that the wonders of the human spirit are expressed in art and science, and I see no conflict between the two. Immersion in the great human achievements of all ages helps me in times of despair and doubt. Human meanness and delusion then no longer seem so important. Honesty, especially intellectual integrity, courage, and kindness are still the virtues I admire; however, with age the emphasis has shifted a bit – kindness seems more important to me today than it did in my youth. Love of my work and dedication to it are the basis of happiness for me.”
You can download this article in German here:
Literature:
- https://cfmedicine.nlm.nih.gov/physicians/biography_69.html
- https://brockhaus.de/ecs/enzy/article/gerty-cori
- www.nlm.nih.gov/changingthefaceofmedicine/physicians/biography_69.html
- https://de.wikipedia.org/wiki/Gerty_Cori
- https://www.fembio.org/biographie.php/frau/biographie/gerty-cori/
- https://www.jewiki.net/wiki/Gerty_Cori
- https://jwa.org/thisweek/dec/10/1947/gerty-theresa-radnitz-cori
- https://www.nndb.com/people/144/000128757/
- https://www.biologie-seite.de/Biologie/Gerty_Cori
- https://study.com/academy/lesson/gerty-theresa-cori-biography-discovery-accomplishments.html
- https://www.nobelprize.org/prizes/medicine/1947/cori-gt/biographical/
- https://www.chemie.de/lexikon/Gerty_Cori.html
If you find any errors or if you are the copyright holder of an image and do not agree to its use on this site, please contact beam@chemie.uni-halle.de.
Agnes Pockels
(1862 – 1935)
“… I could not publish my results directly…”
picture generated by Gesine Born with AI
prompt: wet plate photografie of https://s.mj.run/FwuGjZkA6jA as a 80 year old female standing confidently with one hand on her hip against a dark velvet curtain, foto from 1900, leica style, Lomographic Effekts, film gain, depth of field –s 250 –v 5.2
Agnes Pockels was born on February 14, 1862. She first lived with her parents in Troppau, as her father was stationed there as a member of the army. Later the family moved back to Braunschweig, in keeping with their origins in the Harz Mountains. At the age of ten, she passed the entrance examination for the Städtische Höhere Tochterschule, from which she graduated after five years in 1877. She was taught mainly languages, religion and needlework.
Read more…
At least there was a ray of hope with two hours of natural history lessons per week and even physics lessons in her final year. Physics was of big interest for her. Electricity, light, heat, sound and magnetism filled her to the core and she would have loved to study physics. But her school career was over after the five years. It was not until almost 20 years later that the first girls were allowed to take the Abitur in Berlin as external students. Access for women to equal education was not yet possible. She had to devote herself to domestic duties and care for her parents. Her sister-in-law writes about this: “…what millions of women see every day with unwillingness and are busy cleaning away – the greasy washing-up water – that stimulated this one to observations and finally to scientific work on some questions.”
But she had an ally. Her brother Friedrich, called Fritz, three years her junior, began studying physics in Braunschweig in 1883. He was her equal in conversation and took her physics interests seriously. He provided her with technical literature and was available as a discussion partner. Fritz then changed his place of study and went from Braunschweig to Göttingen, earning his doctorate in Göttingen in 1888. After his habilitation in 1892, he followed a call to Dresden four years later. From 1900 he was professor of theoretical physics in Heidelberg. Even before her brother began his studies, Agnes had, while washing dishes since 1881, systematically studied the changes in the water surface caused by the immersion of solid bodies and dirt. At the age of 20, she wanted to know more and invented an apparatus, the slide trough. With this apparatus, built from simple materials, she was able to produce defined water surfaces. Then, with the help of a suspended beam balance, she could measure the force that had to be applied to tear off small buttons. The slide trough was later named the “Pockels trough” and then, in its more advanced form, was called the Langmuir-Pockels balance. The more evolved sliding trough is still used today for the quantitative study of surface films.
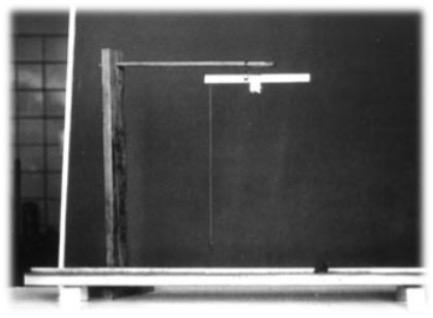
Irving Langmuir received the Nobel Prize in Chemistry in 1932 for his work in the field of surface chemistry, in particular adsorption at phase interfaces. There were the following comments:
“When Langmuir received the Nobel Prize for Chemistry in 1931 for his work in investigating monolayers on solids and on liquids, part of his achievement was thus founded on original experiments first made with a button and a thin tray, by a young lady of 18 who had no formal scientific training.” She recorded her results in a clear, structured, comprehensible and careful manner. Her diary, on the other hand, states succinctly: “1880 or 81. discovered the anomalous behavior of the water surface; 1882. invented slide trough (trough); 1883. had large slide trough made.”
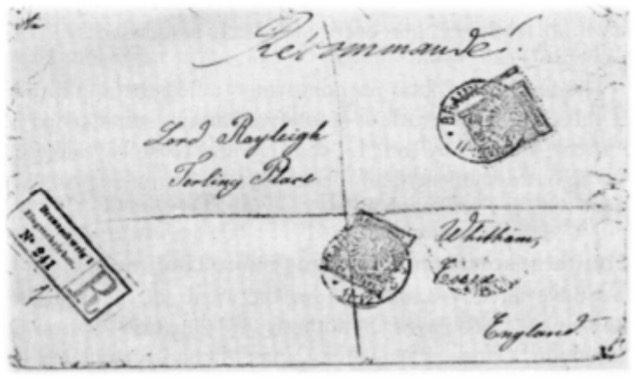
Agnes Pockels communicated her observations in letters to professors at Göttingen University; however, these letters were not heeded, and publication in journals proved more than difficult:
Agnes Pockels communicated her observations in letters to professors at Göttingen University; however, these letters were not heeded, and publication in journals proved more than difficult: “… I could not publish my results directly, partly because the local journals would probably not have accepted anything from a lady, partly because I was not sufficiently informed of the work of others on the same subject. of others on the same subject.” In 1890, she came across a report on work by Lord Rayleigh on the film formation of olive oil on water. A distinguished English scientist who wrote about the same observations as she did and whose calculations agreed with her results. She decided to write him a letter. The letter is dated January 10, 1891, and begins, “Would you kindly excuse my bothering you with a German letter on scientific matters. Having heard of the fruitful work you did last year on the hitherto little understood properties of water surfaces, I thought you might be interested in my own observations on this subject. For various reasons I am unable to publish these observations in scientific journals, and I therefore choose this way to communicate to you the most important of them.”
In her letter, with the matter-of-factness of a scientist, she presented her observations, results and the conclusions drawn from them. The data of several years of work are here precisely summarized and put into a written form. Lord Rayleigh recognized the value of the data and replied to her. An exchange of letters began. But not only this, he saw to it that her work was recognized in the journals of science.

On March 12, 1891, her letter appeared in Nature under the title “Surface Tension.” He could also have used the data for himself, because Agnes Pockels had added to her letter “By the way, I leave it entirely to you to dispose of my little work and to make any use of my communications ….”
Lord Rayleigh, however, enclosed a letter of recommendation with the publication “I shall be obliged if you can find space for the accompanying translation of an interesting letter which I have received from a German lady, who with very homely appliances has arrived at valuable results respecting the behavior of contaminated water surfaces.” Research and curiosity was her passion so she continued to study the adhesion of various liquids to glass, interfacial tensions of emulsions and solutions, and the surface forces of monomolecular films. She continued to publish her results in Nature, the Naturwissenschaftliche Rundschau and the Annalen der Physik. Now the Göttingen professors who had ignored her letters also take notice. The Göttingen professor Woldemar Voigt offered her the use of the Physics Institute in 1893. But her parents prevented this; she was needed at home. She was caught in the social ideas and parental clutches of the time. Her brother Fritz died in 1913, thus she lost access to literature and science. Nevertheless, a number of German-language scientific works appeared between 1914 and 1933. Agnes Pockels was never married and remained childless. She was heavily involved in providing for her parents throughout her life. However, despite these circumstances, she did not become discouraged and pursued her research continuously and with meticulous accuracy. She made fundamental observations and the findings from them endure to the present day. She was courageous. She sought the way into the professional world in order not to withhold her findings from it. Eventhough she received rejection at first, she took the opportunity to write to Lord Rayleigh when it arose, which led to a very positive turn with much inspiration for her research.
In 1931 she was awarded the Laura R. Leonard Prize of the Colloid Society. With the prize money, which she did not use for herself, she supported travel grants and made it available for membership dues of members who could no longer pay. The effects of the Great Depression were still showing. She also subscribed to the Kolloid-Zeitschrift, which, after she read it, was given to the library of the Technische Hochschule Braunschweig.
Her sister-in-law recalls, “She was less affected by it [the war and inflation] because the American relatives tirelessly provided, … . For years she helped and communicated on all sides. She herself always remained the simple-living one who thought her own thoughts but didn’t say much.” Two of her father’s brothers had emigrated to St. Louis, Missouri, as engineers.

She was awarded an honorary doctorate from Brunswick Technical University in 1931. When asked as a result of the honors what she was most pleased about she said, “about the thought that the realization of this fundamental law arose at the same time in Germany, France [Henri Edgard Devaux] and England [Lord Rayleigh].”
She was awarded an honorary doctorate from Brunswick Technical University in 1931. When asked as a result of the honors what she was most pleased about she said, “about the thought that the realization of this fundamental law arose at the same time in Germany, France [Henri Edgard Devaux] and England [Lord Rayleigh].” Compared to the domestic situation, Agnes Pockels’ scholarly achievements were more fully appreciated abroad during her lifetime and after her death. Today, a doctoral award from the Bunsen Society bears her name. The TU Braunschweig awards the Agnes Pockels Medal and there is a student laboratory there named after her.
You can download this article in German here:
Literature:
- Chemikerinnen – es gab und es gibt sie, Gesellschaft Deutscher Chemiker, 2003
- CHEMKON 2012, 19, Nr.2, 78- 82, Petra Mischnick, DOI: 10.1002/ckon.201210173
If you find any errors or if you are the copyright holder of an image and do not agree to its use on this site, please contact beam@chemie.uni-halle.de .
Cecilia Payne-Gaposchkin
(1900 – 1979)
“From a very young age I decided to do research and was seized with panic at the thought everything might be figured out before I was old enough to start.”
picture generated by Gesine Born with AI
prompt: black and white full body portrait of https://s.mj.run/YLwmmZVh0L4 as a 50 year old female scientist, standing confidently with one hand on her hip against a black backround, hard light, foto from 1930, hssselblad 80mm, film gain –v 6.0 –s 250
Cecilia Payne was born in Wendover, near London, on May 10, 1900. As a child, her interest was in botany, in particular the taxonomic classification of plants. To classify something according to certain criteria will later make her a pioneer of astrophysics. Her father, a lawyer, historian and passionate musician, died when Cecilia was four years old. Her mother took care of the family alone. Cecilia’s school career began at a private school. When she was twelve, the family moved to London so that her brother could receive a better education.
Read more…
Cecilia then attended St. Mary’s College until she was expelled in her senior year. She had been found out by having her Plato book bound into the cover of the Bible and pretending to read the Bible in class. She had created her own religion, science. She was then able to attend the last year of school at St. Paul’s Girls’ School. A stroke of luck, because here she was encouraged to think scientifically. “I will never be lonely again! Now I can think about science.”
To be admitted to study at Cambridge at Newnham College, she took an entrance exam, which she passed. This was an all-girls college with its own laboratories. She enrolled in botany, physics, and chemistry in 1919 and also snagged a scholarship. When she heared a lecture by astronomer Arthur Eddington, who sought to verify Einstein’s theory of relativity by observing the eclipse of the sun in Africa, her interests changed permanently: “For three nights, I think, I did not sleep. My world was so rocked that I suffered something like a nervous breakdown.”
From then on, physics was her passion. She researched herself with Ernest Rutherford in the lab. Rutherford found women in physics inappropriate and let Cecilia Payne feel it. She was subjected to discrimination, which she endured, wanting to explore physics.
“I had left the world of dreams and entered reality… Abstract study was a thing of the past; now I was moving among the stars.”
Cambridge did not award degrees to women until 1948, so she finished her studies without a title. It was assumed at the time that women would work as teachers, not scientists. Due to the limited opportunities in England to work scientifically as a woman, she applied for a fellowship from Harvard College Observatory and used it to move to the United States in 1923. The stipend was only barely enough to live on. Harvard had a practice of paying women poorly, if at all, not in scientific positions but as research assistants, and of using them to evaluate stellar spectra. She began a Ph.D. with Harlow Shapley, who wanted to establish his own astronomical department and needed a successfully completed dissertation to do so. She received her doctorate from Radcliffe College in 1925.

She submitted her dissertation with the title: “Stellar Atmosphere: A contribution to the observed study of high temperature in the reversing layers of stars.” She was the first person to receive a Ph.D. in astronomy from Radcliffe College, Harvard University. In her work, she was able to show that the stellar spectral lines measured were not due to the different elements, but to ionization at different temperatures.
She related the spectral classes of stars to their temperature and thus derived their composition. At that time, the conventional scientific opinion was that the stars had a composition similar to that of the Earth. Using her approach of temperature-dependent ionization, she was able to show that stars were composed mainly of hydrogen. This made hydrogen the most abundant element in the universe. She checked her calculations several times and they were correct. However, under pressure from her supervisor Henry Norris Russel, she had to add the sentence “pretty sure it’s not real” to her doctoral thesis. Which she regretted for a long time, because Russel showed in 1929 in independent measurements that her statements were correct. Only then was this fact accepted by the scientific community.
“Work with love, embrace the unexpected, don’t let anyone else make intellectual decisions for you, and always stay in direct contact with the original source,” is an advice she would later give to young women scientists.

Cecilia Payne became an American citizen in 1931. On a research trip through Europe in 1933, she met astrophysicist Sergei Gaposchkin. They married in 1934 and she added his last name to hers.
Her husband took care of their three children while she advanced her career. From 1938 she lectured, but was not mentioned in the Harvard lecture schedule until 1945. “The reward of the young scientist is the feeling, the thrill, of being the first person in the history of the world to see anything or understand anything. Nothing can compare with that experience. The old scientist’s reward is the feeling of having seen a vague sketch grow into a masterful landscape.”
In 1956, she became the first woman to receive a full professorship at Harvard University. She became the head of the Institute of Astronomy, also making her the first woman to head an institute at Harvard. Astronomer Otto Struve called her 1962 doctoral dissertation “the most brilliant work ever written in astronomy.” “I reached a height that I could not have imagined in my wildest dreams 50 years ago. It was a case of survival, not of the fittest, but of the most tenacious. I didn’t consciously aim for the point I finally reached. I just plodded on, rewarded by the beauty of the landscape, toward an unexpected destination.”
She retired from active teaching in 1966 and continued her research as a scientist at the Smithsonian Astrophysical Observatory. She also still served as an editor for scientific journals and the Harvard Observatory’s book publications. Shortly before her death, she had her written autobiography privately printed “The Deyer’s Hand.” The 1984 reprint is titled “Cecilia Payne-Gaposchkin: an autobiography and other recollections.”
She died on December 7, 1979.
You can download this article in German here:
Literature:
- https://scientificwomen.net/women/payne-gaposchkin-cecilia-77
- https://www.aps.org/publications/apsnews/201501/physicshistory.cfm
- https://www.themarginalian.org/2017/07/26/cecilia-payne-gaposchkin-autobiography-advice/
- https://www.themarginalian.org/2019/05/08/cecilia-payne-harvard-observatory-radio-talks/
- https://de.wikipedia.org/wiki/Cecilia_Payne-Gaposchkin
- https://www.amnh.org/learn-teach/curriculum-collections/cosmic-horizons-book/cecilia-payne-profile
- https://en.wikipedia.org/wiki/Cecilia_Payne-Gaposchkin
- https://www.swr.de/swr2/wissen/cecilia-payne-die-astronomin-die-herausfand-woraus-sterne-gemacht-sind-swr2-wissen-2020-11-24-100.html
- https://www.faz.net/aktuell/feuilleton/buecher/rezensionen/sachbuch/biographie-ueber-die-astrophysikerin-cecilia-payne-gaposchkin-16832705.html
- https://www.deutschlandfunk.de/cecilia-payne-gaposchkin-die-frau-fuer-wasserstoff-und-100.html
- https://www.deutschlandfunk.de/cecilia-payne-gaposchkin-100.html
- https://www.britannica.com/biography/Cecilia-Payne-Gaposchkin
If you find any errors or if you are the copyright holder of an image and do not agree to its use on this site, please contact beam@chemie.uni-halle.de .
Dorothea Erxleben
(1715 – 1762)
“The contempt for scholarship is especially evident in the fact that the female sex is discouraged from studying.”
picture generated by Gesine Born with AI
prompt: black and white portrait foto of https://s.mj.run/_k_wDoZDv68 as a 30 year old female scientist with red hair and clothes from 1730 standing confidently with one hand on her hip against a dark background –s 50 –v 6.1 –style raw – Upscaled (Subtle) by @Bilderinstitut (fast)
Dorothea Erxleben was born Dorothea Christiana Leporin in Quedlinburg on November 13, 1715, the second child of four children in the Leporin family. Her father was a physician. Although he had acquired his education to a large extent autodidactically, he completed this with a doctorate at the University of Erfurt. The father taught his eldest son himself, but involved his daughter Dorothea. She was an inquisitive child and enjoyed learning. She overcame her frequent illnesses more easily through mental distraction and intellectually challenging content.
Read more…
The father also instructed his children in the theory and practice of medical content. Thus, his children went with him on house calls and cared for the sick under his guidance.

When Dorothea’s older brother had to serve in the military, the father continued his daughter’s education and supported her in her efforts to obtain a sound education in the medical field. Dorothea was also supported by the rector and conrector of the academic high school in Quedlinburg.
It was also the rector who told the 16-year-old Dorothea in a letter about a woman’s doctorate at the University of Bologna and suggested a similar goal to her. A first step, therefore, was to attend the academic Gymnasium, which, compared to today, can be classified between a grammar school and a university. Thanks to the opportunity to go to the Gymnasium and the special encouragement from her father, as well as her own inquisitiveness and autodidactic acquisition of knowledge, Dorothea had a level of knowledge equal to that of a medical student.

Therefore, she endeavored to pass the official medical examinations and obtain permission from the university to practice medicine, and was granted permission to do so. In 1738 she wrote down her opinions, impressions and thoughts about women and education. Her father discovered the writing four years later and encouraged its publication, initially against Dorothea’s will.
The work was published with the title “Gründliche Untersuchung der Ursachen, die das Weibliche Geschlecht vom Studieren abhalten, darin deren Unerheblichkeit gezeiget, und wie möglich, nöthig und nützlich es sey, Daß dieses Geschlecht der Gelahrtheit sich befleisse, umständlich dargeleget wird” (Thorough examination of the causes that prevent the female gender from studying, in which their irrelevance is shown, and how possible, necessary, and useful it is that this sex should take care of itself, is explained in detail.). The work is divided into two parts.

The first part shows prejudices that prevent female education. The second part presents all the other causes of non-education, such as avarice, envy, and fear.

The work cites many scholars from ancient times to the year of writing, and thus should lead in the reader to the self-reflective conclusion that there is no excuse for non-education, regardless of gender. It advocates equal access for all people to education and more specifically still academic education and activity as a necessity. It is also an appeal to all women to use and exploit the possibilities of education. For Dorothea, a woman’s education is not in conflict with being a mother. When her cousin died, she took over her five children and raised them. A year later, she married the widower and deacon Johann Christian Erxleben and had four more children with him. She lovingly raised all of the children, as evidenced by quotes from the children. She also took on the duties of a pastor’s wife. The diverse tasks did not keep her from continuously expanding her knowledge and skills in the medical field. Her father’s health problems also required increased involvement on her part in his practice. When he died in 1747, she continued the practice on her own responsibility. Her practice was so well attended that the other physicians in private practice regarded her as economic competition and reported her rival to the chapter governor on February 5, 1753. Since Dorothea Erxleben did not have a license to practice medicine, she was operating in a gray area with her practice and was formally liable to prosecution. She responded to the complaint with a 16-page statement in which she announced her doctorate, referring to the king’s permission of 1741. In addition, she offered the complaining doctors to let them examine her. However, they refused. She was then to submit her doctoral thesis within a three-month period. However, the deadline had to be postponed due to the birth of her fourth child on April 14, 1753. What had happened in her life since the request to the king to be allowed to do a doctorate. She had married and adopted 5 children, in the meantime had three children of her own, the fourth was on the way, and had taken over her late father’s practice. Now it was time not to postpone the doctorate any longer, for it was necessary in order to counter the accusations.
On January 6, 1754, she submitted her dissertation to the collegiate governor and asked that it be forwarded to the king. The latter gave his approval for the doctorate on March 6, and this decision was simultaneously communicated to the medical faculty and the collegiate governor in Halle. Since the answer of the university in Halle was long in coming, the collegiate governor wrote to the medical faculty at the end of April and recommended without reservation the doctoral project of Dorothea von Erxleben. He made clear here the urgency of admission to the examination. The professors of the medical faculty agreed, and so on May 6, 1754, Dorothea Erxleben became the first woman to take a medical exam at a university in Germany. The examiners communicated to the king Dorothea Erxleben’s exceptionally good achievements in the theory and practice of medicine and the Latin language.
Her dissertation, written in Latin, was so well received that she translated and expanded it into German. She also reformulated the work so that it could be read and understood by less medically literate members of the population. The work was printed in 1755 with the title “Academische Abhandlung von der gar zu geschwinden und angenehmen, aber deswegen öfters unsichern der Krankheiten”. The work also aimed to counter the “miracle healers” without any medical knowledge, some of whom used methods that caused more harm than good.
More and more patients came to her medical practice after graduation. She was particularly successful in healing women and children. She is also said to have been the personal physician of the abbess. It was to take another 150 years before the next female doctor was able to earn her doctorate.
Dorothea Erxleben died on June 13, 1762.
You can download this article in German here:
Literature:
- Brigitte Meixner, Städtische Museen Quedlinburg – Schloßmuseum, Klopstockhaus, (1999), Dr. Dorothea Christiana Erxleben, Band III, Quedlinburg
- Brigitte Meixner, Städtische Museen Quedliburg – Klopstockhaus, Dr. Dorothea Christianan Erxleben, ein ganz normales Ausnahme-Leben, Band VIII, Quedlinburg
- https://www.sachsen-anhalt-lese.de/index.php?article_id=249
- https://www.kinderzeitmaschine.de/neuzeit/absolutismus/lucys-wissensbox/wissenschaft/wer-war-die-erste-deutsche-aerztin/
- https://www.aerzteblatt.de/archiv/137624/Dorothea-Christiane-Erxleben-Doktor-der-Arzeneygelahrtheit
- https://www.deutsche-biographie.de/sfz13693.html
- https://www.fembio.org/biographie.php/frau/biographie/dorothea-christiane-erxleben/
- http://harzklinikum.com/d/index.php?id=769
- https://frauenorte.net/frauenorte/geburts-und-sterbehaus-dorothea-erxleben-in-quedlinburg/
Image credits:
- Martin-Luther-Universität Halle-Wittenberg, Archiv: US Halle Rep.29 FIII, Nr. 2, bd. 5
The remaining images are in the public domain. If you are the copyright owner and do not agree with the use on this website, please contact beam@chemie.uni-halle.de.
If you find any errors or if you are the copyright holder of an image and do not agree to its use on this site, please contact beam@chemie.uni-halle.de .
Erika Cremer (1900 – 1996)
“It has actually always given me pleasure to solve a scientific problem”.
picture generated by Gesine Born with AI
prompt: Photograph 60 yer old female scientist of https://s.mj.run/LE-aB0Odirs , sitting in a chair, wearing formal suit attire, in a black and white photograph, facing forward, a headshot portrait, in a vintage photography style, with a classic composition, balanced lighting, and a historical background, conveying a timeless elegance and iconic pose –s 250 –v 6.1
Erika Cremer was born on 20.05.1900 in Munich. Her father, himself a professor as well as her great-grandfather and grandfather, motivated her early on to pursue an academic career: “Learn something, study, that’s the only fortune that even the worst political events can’t take away from you.”
Read more…
She recounts the following from her school career: “I can remember that in the first elementary school class I was pulled out by a teacher, to the front, which usually meant something bad, but in this case she actually wanted to know something quite good from me. She wanted to know what I wanted to be when I grew up. That was still a very modern question at that time, because girls were not supposed to become anything, they were supposed to stay at home, were then supposed to set up a household – spend their whole lives in this environment. But I was absolutely not embarrassing to say what I had to say, namely: ‘I want to become a student.”
In 1911, the family moved to Berlin for the father’s professional reasons. In 1920, Erika Cremer took her Abitur here at a study institute for young women. She began her studies in chemistry, physics and mathematics immediately afterwards. Her father drew her attention to Nernst’s lecture Introduction to Chemistry: “Nernst is reading an introduction to chemistry, you have to hear it.”
Ms. Cremer said of her career, “… my personal career, it was already a bit prescribed. And it was dictated by the fact that I was born just at the time when women were allowed to go to university and study. And my father was very much in favor of that and actually steered me in that direction early on. The brothers, of course, played more of a negative role. The brothers very much said they were extraordinarily convinced of their masculinity and the merits of that masculinity, and when I somehow wanted to know something, my brother, who later became a mathematician, by the way, would say, “You wouldn’t understand, you’re just a woman.” But in doing so, they had the opposite effect. They just stimulated pride in me to show you that I do understand. I think they have as much part in my having studied as my father, who advised me.”

She did her doctoral thesis with Max Bodenstein from physical chemistry. The topic was on the chlorine oxyhydrogen reaction and that a chain reaction determines the conversion rate. She finished her doctoral thesis in 1927.
The data and conclusions were so new and daring that Prof. Bodenstein left the publication to her with the remark: “you did it all yourself”. Her results found recognition with Nikolai Semenov, who was doing research in the same field in Leningrad. He invited her for a research stay, but she declined. Semenov received the Nobel Prize in Chemistry in 1956 for research and elucidation of chain reactions. Actually, Erika would have been entitled to the prize for her doctoral thesis, she confidently believes.
After receiving her doctorate, she spent 10 years working as an unpaid or poorly paid employee. A doctorate in chemistry by a woman in those times hardly brought any significant advantages on the labor market. She worked unpaid at the Kaiser Wilhelm Institute (KWI) for Physical Chemistry in Dahlem from 1930-1933 and at the KWI for Chemistry in 1937, also unpaid. In 1933, the working group in Dahlem at the KWI was disbanded under director Fritz Haber. “At first we thought we might be able to continue the institute after all, because Haber was very well credited by name with the military, …’Yes, if Haber can stay, then all he has to do is say that he can’t work without the others.’ And they have to stay now, too. Then we can keep the whole institute.'” However, this plan did not work out. “People put pressure on Haber in such a way that Haber said: If you don’t let my employees stay, then I’ll go too. He thought that this would mean that he, Haber, was worth so much to them that they would let his staff stay, but it was the other way around: ‘Then you go’. And Planck [as president] could do nothing more, and all of a sudden it was said that the whole institute had to go.”
Erika Cremer was also dismissed, and she subsequently had to change her research direction several times. With a research grant, she worked on alcohol decomposition on rare earth oxides at Hevesy in Freiburg. “I noticed in my measurements that there was a formulaic describable dependence between the heat of activation and the activity of the catalyst, the catalytic reaction.” This became known as the “compensation effect.” However, it was later shown, also by Cremer, to be a superposition of various experimental factors. Cremer said of this, “I was skeptical from the start and at least added in the title still, about a possible explanation.” She most likely enjoyed her stay in Freiburg very much, as she loved the mountains and had missed them since leaving Munich. She then worked with Michael Polanyi and with Kasimir Fajans at the Physikalisch-Technische Reichsanstalt (PTR). Here at the PTR, circumstances due to a president who was very close to the National Socialists required Erika Cremer to hide from him. For he was not allowed to know that there was a female scientific employee at his institute. This period was followed by work at the bioclimatic research institute of Kiel University. “And then I came back to Berlin and then people took pity on me. And then Bodenstein arranged for Hahn to take me into his institute. And so, I came to Hahn’s institute [19]37 and was also [19]38 there, in the institute how the great discovery was made.” Erika Cremer worked for the greats in her field, because here she felt she was “in the middle of the scientific front.”
In 1938 she habilitated at the University of Berlin on the “Determination of self-diffusion in solid hydrogen from the reaction course of the ortho-para conversion.” The following statement is handed down from the dean: “We will give you the Dr. habil. but you will never get a lectureship.” Two years later in 1940 she accepted the call to a lectureship at the University of Innsbruck. Here in the mountains she could ski again and enjoy nature, to which she felt connected in her innermost being.
The lectureship was linked to the fact that she had to give it up again at the end of the war. However, things turned out differently and at that time she was offered the directorship of the Physical-Chemical Institute in Innsbruck. In the explanatory statement it says: “Ms. Cremer is also developing a very fruitful teaching activity and has already successfully guided about a dozen doctoral students to the doctorate. She enjoys great popularity among all listeners, and her lectures are well attended. Similarly, cooperation with colleagues is developing as a result of her open-mindedness and helpfulness.” This was a higher position than she could have imagined after her doctorate: “… this “post-doctoral study” – I was able to
make great use of it and actually did not expect more, even for later, than that I could perhaps become a lecturer, with one or two co-workers and be allowed to work on some interesting problem there.”
While she was still a lecturer, she had already worked experimentally on the hydrogenation of acetylene. Her theoretical considerations were concerned with the question of whether it would be possible to separate small proportions of acetylene and ethylene by means of adsorption. Using mathematics, she was able to establish a relationship between adsorption energy and retention time.
These considerations, which anticipate the analytical application of gas chromatography she compiled for publication. The proofs for which have been preserved to this day, but the end of the war prevented delivery of the journal “Naturwissenschaften” and so this work was not published. After the war, the German Erika Cremer was considered a foreigner in Innsbruck and could no longer travel to her workplace, the institute 15km away, due to the exit clearance of 3km. It is known that she was brought in a closed van to her co-workers in the heavily destroyed institute.
In November 1945, Fritz Prior began a doctoral thesis, which he was able to master alongside his job as a teacher. Erika Cremer proposed a chromatographic separation of gas mixtures. This dissertation showed that the theoretical considerations for the analysis were correct. In 1951 she was appointed associate professor and also head of the institute. “A colleague once said to me, ‘Well, I wouldn’t work so much in your place after all if I had as little chance as you do of ever becoming a professor.’ To which I replied to him, ‘I’m not doing it to become a professor either, I’m doing it because I enjoy it.’ Equality of opportunity certainly didn’t exist, but I would say we realized that it couldn’t exist immediately. And when you have achieved it, then of course you are happy. But then, when you have achieved it, I have to say that I have actually had no more difficulties. I have been recognized, both by students and colleagues, and I cannot complain that I have been treated worse than a male colleague.”
She described the competitive situation with her male colleagues as follows: “As long as you studied, it went quite well. But when you had finished and were a competitor, you realized that you had much fewer opportunities than the men. Incidentally, we actually realized ourselves that this had to be the case. First of all, we were a kind of “new intruders” who were now entering a business that had previously been run only by men; and then the men also wanted to start a family and wanted to have a permanent position to do so. I never had a permanent position for more than 10 years after I got my doctorate, which I did in 1927.”
32 years after completing her doctorate, she is finally appointed a full university professor of physical chemistry. At the age of 70, she retired from this position. She was honored many times for her achievements, e.g. with the honorary doctorate of the TU Berlin, the American Tswett Medal and the Tswett Medal of the USSR and the Bunsen Denkmünze.
In 1955, she gave schoolgirls the following advice: “May she stand on her own two feet. Be independent, not be a burden to parents or siblings. Independent in the choice of a husband, not having to marry out of ‘provision’, which was the fate of almost most women in earlier centuries. … After all, marriage is not a life insurance either. How many women suddenly had to take over the care of the family during the war or after the war. … Development. Everyone needs a goal. Everyone needs something that fills him. You can’t imagine a mature personality without a profession that corresponds to his real vocation.”
“She loved chaos, entropy was always a popular term. She had ideas, she was brilliant, that was what made her strong.”
A habilitation program at the University of Innsbruck is named after Erika Cremer. She supervised 71 doctoral students and 4 habilitation students during her time at Innsbruck.
You can download this article in German here:
Literature:
[1] Erika Cremer, Pionierin der Gaschromatographie, Klaus Beneke
[2] In memoriam em. Univ.-Prof. Dr. phil. Dr. rer. nat. H.c. Erika Cremer, 96 Jahre eines Forscherlebens von Ortwin Bobleter, Innsbruck, Okt. 1997
[3] „Chemikerinnen – es gab und es gibt sie“, Arbeitskreis Chancengleichheit in der Chemie (AKCC), 2003
[4] https://physik.cosmos-indirekt.de/Physik-Schule/Erika_Cremer
If you find any errors or if you are the copyright holder of an image and do not agree to its use on this site, please contact beam@chemie.uni-halle.de .
Gertrude Belle Elion
(1918 – 1999)
“I wanted to learn everything, everything I saw. What I learned in school never satisfied me.”
picture generated by Gesine Born with AI
prompt: Vintage foto from 1948 from 30 year old female scientist https://s.mj.run/he4nmn4l_5I standing confidently and proud and serious against a black backround with crossed arms –v 6.1 –s 80 –style raw
Gertrude Belle Elion was born in New York on January 23, 1918. She had a brother six years younger. Her parents emigrated to the United States from Poland and Lithuania when they were children themselves. Gertrude Elion’s father was a dentist and the family lived in a large apartment in Manhattan during Gertrude’s first seven years of life. The father’s practice was also located in this apartment.
Read more…
The family then moved to the Bronx, at that time still a suburb of New York with many parks, a zoo, and plenty of room to play outside due to undeveloped land. The siblings went to a public school within walking distance of their home. Gertrude had an irrepressible thirst for knowledge and found all school subjects equally interesting. Therefore, she was in a dilemma when she had to choose a major for further specialization after high school. When she was 15 years old, her maternal grandfather died of cancer. That tipped the scales in favor of choosing a major in science. She later wanted to develop something that helped against this disease. She then went to Hunter College beginning in 1933, specializing in science and majoring in chemistry. The college she chose was also a public school. At that time, there were already many fee-paying private schools and the public schools chose their students based on grades. Since this was already considered a secondary education and her father had lost a lot of money in the economic crisis of 1929, it was very fortunate that her grades were so excellent that she was able to attend college. Otherwise, she is quoted as saying, she probably never would have gotten a higher education. The college was an all-girls college. Some of the girls later became teachers and others went into science. Because of the Depression, however, she was unable to go directly to college. She applied for assistantships and scholarships, but got none of the few available. Jobs were also scarce and positions in laboratories were not open to women. She found a position teaching biochemistry to nurses in 1937.

However, due to the trimester rule, it was only for three months and then that position was not available for the following nine months. “No one took me seriously. Everyone asked why I wanted to be a chemist when no other women wanted it. The world wasn’t waiting for me.” But an opportunity to break into research was approaching. Gertrude Belle Elion met a chemist who was looking for a lab assistant, and although he couldn’t pay her, she accepted the position because she expected to learn a lot. She stayed in the position for 1 ½ years and pretty soon was earning $20 a week. She was able to put some of that away, and with the help of her parents, she began studying chemistry at New York University in 1939. She was the only woman in her semester, but no one noticed this strangely and she didn’t find it strange at all. After only one year, she had already completed all the necessary courses and was looking for a topic for her master’s thesis, which she worked on at night and on weekends.

That’s because during the day she had a position as a teacher in training and later as a substitute teacher in high schools in New York for the subjects of physics, chemistry and general science.
Despite this double burden, she completed her master’s thesis in chemistry in 1941. By this time, World War II had begun and chemists were in short supply in industry. Although this was the case, Gertrude Elion did not find a position in research. However, instead in an analytical food laboratory where she worked from 1937 – 1938. After 1.5 years, she was so bored by the activities because she was not learning anything new that she reapplied and accepted a position as a teacher in a high school from 1940 -1942. She then worked as a research chemist at Johnson and Johnson from 1943 -44. However, the lab was closed after six months. But after this, a few lab positions were open to her and she chose an assistant position with George Hitchings. She liked this position very much because of the mentor George Hitchings. As he gave her the opportunity to learn a lot and take on more responsibility if she wanted to. Having worked mainly in organic chemistry up to that point, she now moved more and more into the areas of microbiology and investigated the biological activities of the substances she synthesized.
At the same time she wanted to do her doctorate and went to the polytechnic institute in Brooklyn in the evenings. After a few years of commuting within New York, she was informed that she could not continue with the doctoral work if she had the job as assistant to George Hitchings also in parallel. She decided to continue with George Hitchings’ work and stopped doing her PhD. This was a fundamental decision in her life. “My work was fascinating from the beginning. We were exploring new frontiers because very little was known about the biosynthesis of nucleic acids or the enzymes involved.” Later, when she received three honorary doctorates from the universities of Washington, Brown and Michigan, hindsight showed the wisdom of that decision. Her field of research had as its basis the comparison of the biochemical and molecular biological relationships of the healthy human cell with those of a cell suffering from cancer or infected with viruses or bacteria. Based on the results, drugs were then developed which, while damaging the infected and diseased cell and thus driving it to cell death, did not affect the healthy cells. Together with George Hitchings, she developed her first chemotherapeutic agent (diaminopurine) in 1948. This was followed in subsequent years by the chemotherapeutic agent thioguanine (1950) and 6-mercaptopurine in 1951 to treat leukemia. “When we began to see the results of our efforts in the form of new drugs that met real medical needs and benefited patients in very visible ways, our sense of reward was immeasurable.” The drug pyrimthamine for the treatment of malaria was published in 1950. This drug is a diaminopyrimidine, as is trimethoprim, which originated in 1956 to treat bacterial infections. The first immunosuppressant (azathioprine) for organ transplant patients was developed by 1957. Allopurinol became available for the treatment of gout in 1963. The well-known antiviral aciclovir for the treatment of herpes simplex came on the market in 1977. For the treatment of AIDS, the thymidine derivative zidovudine came on the market in 1985. Ms. Elion continued to work on the further development of drugs for AIDS even in her retirement.

During her industrial career, she climbed steadily up the career ladder. In 1967, she became head of the Experimental Therapies Department. She held this position until she retired in 1983. The department she headed was very large and was often jokingly referred to by her colleagues as a “mini-institute” because the department was divided into subunits.
It included a section for chemistry, enzymology, pharmacology, immunology, virology, and a large cell culture section. The resulting close cooperation made it possible to develop this large number of drugs.
She was awarded the Nobel Prize in 1988, along with George Hitchings, “for her pioneering discoveries of important biochemical principles of drug therapy.”
Gertrude Belle Elion has received many honors. In addition to the three honorary doctorates already mentioned, she was also a member of the “American Chemical Society”, the “Royal Society of Chemistry”, the “Transplantation Society”, the “American Society of Biological Chemists”, the “Association for Cancer Research”, of which she was president from 1983 – 84, the “America Society of Hematology”, the “American Association for the Advancement of Science”, the “America Association of Pharmaceutical Scientists” and the “New York Academy of Sciences”. In her retirement, she was a consultant to her previous company and became a professor of medicine and pharmacy at Duke University. During her career as a professor, it was important to her to inspire undergraduate and graduate students to pursue research. She also visited schools and distributed science books to school children.
She was awarded the Lemelson-MIT Lifetime Achievement Award in 1997.
Gertrude Elion was a workaholic. She enjoyed her work so much that she rarely took time to relax. Nevertheless, she enjoyed traveling and was an amateur photographer. She also loved opera and went to the Metropolitan Opera as a subscriber for 40 years. In addition to plays at the opera, she also enjoyed ballet, concerts, and going to the theater. She never married and had no children, but thoroughly enjoyed spending time with her three nephews and one niece. These had children of their own during Gertrude Elion’s lifetime. She felt a close bond with the family, which had grown in number as a result. Despite the great distances between the individual family members, the relatives had a close emotional bond and shared joy and sorrows with each other.
She died on February 21, 1999, in Chapel Hill, North Carolina, in the United States.
„Don’t be afraid of hard work. Nothing worthwhile comes easily. Don’t let others discourage you or tell you that you can’t do it. In my day I was told women didn’t go into chemistry. I saw no reason why we couldn’t.”
You can download this article in German here:
Literature:
- https://www.dpma.de/dpma/veroeffentlichungen/aktuelles/patentefrauen/gertrudeelion/index.html
- https://www.nobelprize.org/prizes/medicine/1988/elion/lecture/
- https://www.nobelprize.org/prizes/medicine/1988/elion/biographical/
- https://www.chemie-schule.de/KnowHow/Gertrude_Belle_Elion
- https://beruhmte-zitate.de/autoren/gertrude-belle-elion/
- https://www.goodreads.com/author/quotes/7793243.Gertrude_B_Elion
- https://www.britannica.com/biography/Gertrude-B-Elion
- https://www.spektrum.de/lexikon/biologie/elion-gertrude-belle/20873
- https://de.wikipedia.org/wiki/Gertrude_Belle_Elion
- https://www.fembio.org/biographie.php/frau/biographie/gertrude-b.-elion/
- https://www.wasistwas.de/archiv-wissenschaft-details/frau-elion-und-die-medizin.html
- https://medicampus.uni-muenster.de/dale-info0.html
- https://www.chemie.de/lexikon/Gertrude_Belle_Elion.html
- https://www.biologie-seite.de/Biologie/Gertrude_Belle_Elion
- https://geboren.am/person/gertrude-belle-elion
If you find any errors or if you are the copyright holder of an image and do not agree to its use on this site, please contact beam@chemie.uni-halle.de .
Hertha Sponer
(1895 – 1968)
“Another point that makes the publication of this work even more urgent (if that is still possible) is the fact that several institutes have taken up this (my original) field of work.”
picture generated by Gesine Born with AI
prompt: Black and white Photograph of 40 yaer old female scientist https://s.mj.run/dufgmj0OlhI wearing a white suit,standing in front of a black backround, Leica style, Foto from 1930 –s 250
Hertha Sponer was born in Silesia in 1895. She attended school in Neisse and Zittau from Easter 1901 to Pentecost 1907. Then she received private lessons at the boarding school in Zittau until Easter 1910. Then, from autumn 1910 to Easter 1912, she attended the women classes in a Gymnasium in Berlin and afterwards she attended the Realgymnasium in Zittau for a quarter of a year. The school in Saxony was very poor in subject matter and about a year behind the Silesian school she had previously attended in learning. As a result, she had put aside her desire to ever attend university and began the seminar as a children educator in Hannover and Heidelberg.
Read more…
She completed her training as a kindergarten teacher in 1913. Immediately afterwards, she worked as a teacher for two years until she went to Breslau in 1916 to attend a “preparatory school” to obtain her Abitur. After her numerous necessary changes of school, she was able to take the Abitur as an external student at a boys’ high school in 1917 at the age of 21. She began her studies in Tübingen in 1917, but transferred to Göttingen in 1918. Where she studied until 1920 and in her last year received her doctorate under Peter Debeye. The title of her doctoral thesis is “On ultrarote absorption of diatomic gases.” In the viva, she was examined in mathematics, physics and chemistry. She then went to Berlin for a year to the Kaiser Wilhelm Institute, the forerunner of the Max Planck Society, where she worked with James Franck. She then went back to Göttingen with him.
Here she worked as an assistant/habilitand at the University of Göttingen from 1921 – 1925. Her habilitation thesis, with which she received her teaching license, is entitled: “Excitation potential of the band spectra of nitrogen”. During this time she established a private seminar with young colleagues at her home; she was the focal point of the young physicists in Göttingen. It was also in this seminar that Heisenberg first presented the draft of his uncertainty relation. After her habilitation, she acquired a Rockefeller Fellowship and went to Berkeley in California in 1925 – 1926. There is an anecdote about this that her colleagues took the liberty of seeing her off with a large crowd at the Göttingen train station. They also sent an article about it to the Göttingen daily newspaper, which was printed. After her stay in Berkeley, she became a private lecturer in Göttingen for seven years, and in 1932 she was appointed an associated professor.

They also sent an article about it to the Göttingen daily newspaper, which was printed. After her stay in Berkeley, she became a private lecturer in Göttingen for seven years, and in 1932 she was appointed an associated professor.
For in 1932, after publishing “Remarks on the Predissociation of Triatomic Molecules,” she could no longer be ignored. This meant the title of professor, but tenure as a full professor, as her male colleagues received, was unthinkable for a woman at the time. The ambitions to make a career as a woman were made almost impossible by the Nazis. Since James Franck resigned his position at the university in protest against the regime, the position at the University of Göttingen was without scientific future for her in perspective. After the Nazis came to power, she no longer saw any scientific prospects for herself in Germany and decided to go to Oslo in Norway in 1934 for a visiting professorship in order to forestall her inevitable dismissal. She then decided to emigrate to the USA in 1936, before her residence permit for Norway expired. In parallel with her stay abroad in Norway and her planned emigration, she published her two-volume work “Molecular Spectra I and II” with Springer Verlag in 1935 and 1936. The first volume is a monograph and the first published textbook on molecular spectroscopy.

The second volume a table work. It introduced in the volumes the classification of atomic and molecular spectra and rotational and vibrational energies. Through this edition she was definitely visible in the first rank of molecular physicists. For the emigration to the USA she had supporters.
It has been handed down that an officer of the Rockefeller Foundation’s program for the support of German scientists forced to emigrate informed the head of the Physics Department at Duke University in Durham that Hertha Sponer might be available for a professorial position. The physics department was in desperate need of competent teaching reinforcements. Hertha Sponer was awarded a full professorship, which she retained until her retirement in 1966. At the time of her appointment, she was the first woman at the Physics Faculty. Here she set up her spectroscopic apparatus in the basement of the institute, which was the quietest and vibration-free place in the building. Moreover, in the basement, the temperature was subject to minor fluctuations.

During her time in Göttingen, Hertha Sponer had already been working interdisciplinary work between the fields of physics and chemistry. She understood and pushed the importance of transferring spectroscopic methods to chemistry. She applied molecular spectroscopy to chemical problems. Her merit was the combination of the fields of chemistry and physics. Later her work was counted as part of the field of physical chemistry, although her range of methods was that of a physicist.

In 1946, she married her longtime friend and mentor James Franck, who by this time was widowed. Both were still very active professionally and James Franck held a professorship in Chicago. Therefore, they both stayed at their respective residences and saw each other only a few times a year for vacations and at conventions. An intensive correspondence has been archived, which proved that Hertha Sponer wrote James Franck not only about scientific topics, but also about everyday circumstances.
After her retirement, she returned to Germany and died in Germany in 1968. Here the sources differ greatly as to whether she was visiting Germany or whether she wanted to spend the evening of her life with her nephew’s family.
You can download this article in German here:
Literature:
- Universitätsarchiv Göttingen, Phil. Fak. Rom. S. Vol. III. 1918-1911, Nr. 9
- https://www.dpg-physik.de/vereinigungen/fachuebergreifend/ak/akc/sponerpreis/wer-war-hertha-sponer
- https://physik.cosmos-indirekt.de/Physik-Schule/Hertha_Sponer
- https://physics.duke.edu/about/history/historical-faculty/HerthaSponer
- Download Excerpt Durden’s Launching of Duke University
- Download King Essay Refugee Scholars at Duke University
- https://www.neues-deutschland.de/artikel/1141160.hertha-sponer-ritterschlag-fuer-eine-ausnahmefrau.html
- https://www.deutsche-biographie.de/sfz123855.html
If you find any errors or if you are the copyright holder of an image and do not agree to its use on this site, please contact beam@chemie.uni-halle.de .
Maria Goeppert-Mayer
(1906 – 1972)
“Watching the solution come out, that was the real fun“.
picture generated by Gesine Born with AI
prompt: leica vintage foto from 1935 of 30 year old female scientist https://s.mj.run/MsYzRR3Tm4s standing confidently, proud and serious in front of a dark background, smoking a cigarette –v 6.1 –s 20 –style raw
Maria Goeppert was born on June 28 in Katowice, Upper Silesia, in what is now Poland. Her father was a professor of pediatrics and already the sixth generation of professors in a straight line of kinship. She was to become the seventh generation. Her mother was a teacher of music and languages. When Maria Goeppert was three years old, the family moved to Göttingen. Her father encouraged her as a young child and it made no difference to him in her upbringing that she was a girl.
Read more…
He would tell her when putting her to bed or on walks, “Don’t ever be a woman when you are grown up.”. This meant she should be different than most women; learn something and not spend her life as a housewife. He said, “You will do it differently, you will study and do something interesting.” She finished elementary school in Göttingen and then took private lessons at a private school run by women’s rights activists, the Suffragette School. This was because girls were not yet admitted to grammar schools. For her parents, it was always clear that she would one day study. “It was never discussed between my parents and me, it was kind of a given that I would go to university. But at that time, it wasn’t that easy for a girl.” She graduated from high school at the age of 17, in 1923, along with four other girls as externs at a boys’ high school in Hannover. After graduating from high school, she began studying mathematics in Göttingen in 1924. Göttingen was the stronghold for mathematics at the time. In 1927, she went to Cambridge, England, for a semester at Girton College. After three years of studying mathematics and her semester abroad, she switched to physics. Solving constructed problems seemed too unworldly to her and more and more like “puzzle solving.” Physics had more practical relevance for her, which was also “like puzzle solving, but they are puzzles created by nature, not the human mind.” She wrote her dissertation under Max Born and received her Ph.D. in 1930, the subject of her dissertation being theoretical considerations of “double photon processes.”
In the same year she married the chemist Joseph Mayer, who lived with her mother as a subtenant. Maria Goeppert’s father had died in 1927 and her mother had started subletting rooms. After the marriage, Joseph Mayer accepted a professorship at Johns Hopkins University. Maria Goeppert went with her husband to Baltimore. The chances to get a professorship as a woman in Germany were still very bad at that time. In Baltimore, no one knew her and no one offered her a position. But she was optimistic: “I was young, I was sure of myself, I knew I was good.” The situation for academic women at the time of the Great Depression was dire in the United States. Again, academia was dominated by men, although women had earlier access to education and colleges in the United States. Professors’ wives were not hired, but were merely given unpaid assistantships or worked for their professors’ husbands without pay. Nepotism prevented them from working independently as paid academics. Maria Goeppert-Mayer accepted a position as a German correspondent for a physics professor. The money she earned went toward hiring a maid. Associated with the position of correspondent was the use of an attic room for physics research.

Here Maria Goeppert-Mayer continued her research in physics out of pure pleasure without paid employment. She was not happy with the situation, but she was not a fighter in this respect either. She did make demands, but her motto was also, “I don’t want to get upset.”
She was thus an unpaid staff member in the Department of Physics at Johns Hopkins University from 1931-39. During this time, her daughter Marianne (1933) and her son Peter (1938) were born.
She took the birth of her daughter as an opportunity to become an American herself. Therefore, she became an American in 1933. She became absorbed in her role as mother, and visits to the physics attic for research became increasingly rare. Her husband brought her back to the desk and together they wrote a textbook on “Statistical Mechanics” in 1938.
She followed her husband locally in his career for life, next moving to New York. They moved to a suburb of Manhattan called Leonia. Here, the academic women were again condemned to academic inactivity. “The women all talked about their babies and the men talked about science.”
She, however, was a lecturer at Columbia University in New York from 1940 – 1946. Here, however, at the Department of Chemistry. She was also a lecturer at Sarah Lawrence College in New York from 1942-1945. From 1941 she worked for the government on the separation of uranium isotopes. As a physicist, she found the scientific and technical uses of uranium isotopes interesting.

She naturally followed the explosives tests in the Los Alamos desert with curiosity as a scientist in this field. But she was dismayed by the ultimate use of nuclear weapons. She had always advocated the peaceful use of atomic power and had publicly joined other scientists in warning against its proposed use.
The family moved to Chicago in 1946 because conditions were better for the couple here. She quickly got a professorship and was a professor at the Enrico Fermi Institute at the University of Chicago from 1946 – 1959, though also without pay. She worked in Chicago at the Institute of Nuclear Physics and at a new government laboratory, Argonne National Laboratory. At the time, Chicago was the center for nuclear physics in the United States. Many nuclear physicists were gathered here and the academic environment was stimulating and exciting. The nuclear physicists were concerned with the structure of atoms. Maria Goeppert-Mayer described the mystery of atomic structure as follows: “No one has ever seen an atom, nor is anyone ever likely to see one. But that does not stop the physicist from trying to draw a plan of the atom, using the clues to its structure that he just has. (…) For the total atom, modern physicists have developed a useful model based on the structure of our planetary system: It consists of a nucleus in the center, corresponding to the sun, and of electrons which move around the nucleus like satellites, like planets on certain, fixed orbits. (…) The nucleus itself, however, is still hardly understood.”
Starting in 1948, Maria Goeppert-Mayer began research on atomic nuclei. Why did some elements occur more frequently than others? Could it be that they had a particularly stable nucleus? “Every nucleus, can be characterized by two numbers: The number of protons and the number of neutrons. The sum of the two gives the atomic weight of the nucleus. The number of protons determines the nature of the atom, a nucleus with two protons is always helium, one with three protons is always lithium and so on. But a fixed number of protons may be combined with a varying number of neutrons, and so different isotopes of the same element are formed. Some isotopes are stable; others decay rapidly by radioactivity.” She wrote down the proton and neutron numbers in tables. “And all of a sudden it came out that in these nuclei either the number of protons or the number of neutrons were very special.” This refers to the particularly stable nuclei of common elements. The “magic numbers” are 2, 8, 20, 28, 50, 82 and 126. It must have been exciting days as Maria Goeppert-Mayer got closer and closer to the puzzle. She explained “spin-orbit coupling” to her daughter: “Think of a room full of dancing couples. Imagine them dancing in a circle, in several circles, one enclosed by the other. In the
same way, electrons circle the nucleus of an atom, one shell enclosed by the next larger one. Then imagine that you get twice as many pairs of dancers in each shell, if one pair moves clockwise, the other counterclockwise. Then think of something else. All the dance couples rotate around themselves like spinning tops as they circulate in space; so each couple rotates around itself and circles in space at the same time. But only some of those who dance clockwise through space also rotate clockwise. And only some of those that dance counterclockwise rotate counterclockwise at the same time. That’s how electrons do it, too.” The difference in energy for an electron spinning clockwise or counterclockwise is tiny. This effect, weak in electrons, should also be the explanation in the nucleus? She discussed her reasoning with Enrico Fermi and it popped into her head. “Why not assume the effect is strong?” She drew only to convince Fermi, in her head she had already finished calculating, “That’s it, Enrico, that’s the solution! Look, everywhere the magic numbers are coming out!” The spin-orbit coupling in the atomic nucleus is much stronger than in the atomic shell. The magic numbers explained it. “I used group-theoretic methods to develop a new classification scheme for atomic nuclei based on a shell model. Atomic nuclei are particularly stable when a nucleon shell is completely filled with a certain number of protons or neutrons. I found that the stability, or instability, depends on the configuration and motion of the protons and neutrons. However, my model can only explain some properties of the nuclei.” She was awarded the Nobel Prize in Physics for this explanation. In 1949/50 she postulated, simultaneously but independently of J. H. D. Jensen, the shell model. The competition between the scientists resulted in a transatlantic collaboration, which led to the joint book “Elementary Theory of Nuclear Shell Structure.”
For developing the shell model of the atomic nucleus, she received the Nobel Prize in Physics in 1963, along with J. H. D. Jensen. The two shared one half of the Nobel Prize. The other half was given to Eugene Paul Wigner for his theory of the atomic nucleus and elementary particles. Of the Nobel Prize, she said, “To receive this prize was an incredible honor for me.” About the Nobel Prize celebration, however, she said, “The only thing I didn’t like about it was that I wasn’t allowed to smoke for hours!”

“Watching the solution come out, that was the real fun.” She thus became the second woman, 60 years after Marie Curie, to win the Nobel Prize in physics. But the first in theoretical physics and also the first U.S. woman. In 1960, the family moved again, and this time to La Jolla, California. Shortly after receiving the Nobel Prize, she suffered a stroke that resulted in permanent hemiplegia.
However, this did not prevent her from continuing to research and publish. She also encouraged young women to pursue a career in the natural sciences. She died on February 20 in La Jolla. Maria Goeppert-Mayer received many awards besides the Nobel Prize. For example, she was elected to the National Academy of Sciences in 1956. In 1950 she became a corresponding member of the Heidelberg Academy of Sciences. From 1965 she was a member of the American Academy of Arts and Sciences. She also received honorary doctorates from Mount Holyoke College, Russel Sage College and Smith College.
You can download this article in German here:
Literature:
- Nobel Lectures, Physics 1963-1970, Elsevier Publishing company, Amsterdam, 1972
- Maria Goeppert-Mayer, Fach Hochschule Lübeck, Physikposter
- www.uni- muenster.de/Physik/department/equality/women_and_physics/history/maria_goep pert_mayer.html
- https://www.nobelprize.org/prizes/physics/1963/mayer/biographical/
- https://www.britannica.com/biography/Maria-Goeppert-Mayer
- https://www.schulmodell.eu/index.php/physik/685-maria-goeppert-mayer.html
- https://kulturstiftung.org/biographien/goeppert-mayer-maria-3
- https://kulturstiftung.org/biographien/goeppert-mayer-maria-3
If you find any errors or if you are the copyright holder of an image and do not agree to its use on this site, please contact beam@chemie.uni-halle.de .
Liselott Herforth
(1916 – 2010)
“Do all the things yourself that you are sure no one else can do better than you.“
picture generated by Gesine Born with AI
prompt: Hasselblad black and white Portrait from 1950 of 30 year old female scientist https://s.mj.run/pwxxRn4IV6s standing confidently, waering buddy holly glasses, standing proud and serious in front of a dark background –v 6.1 –s 10 –style raw
Liselott Herforth was born in Altenburg. However, she spent her early childhood in Eydtkuhnen, where she started school in 1923. A year after starting school, she moved with her parents to Marienwerder, where she also finished elementary school. She attended high school for a year in Leipzig and continued in Berlin. In 1933 she obtained her secondary school-leaving certificate and after a further change of school to obtain her Abitur, she graduated from an all-girls school, a Lyceum, in 1936. For a long time she had toyed with the idea of taking up a profession in the musical direction. Gradually, however, her enthusiasm for the natural sciences, especially physics and mathematics, developed.
Read more…
“Shortly before graduating from high school, a friend who knew my love for the natural sciences brought me a biography of Marie Curie. […] I gained insight for the first time into what research means, including what enthusiasm, determination and perseverance can achieve. My desire to study mathematics and physics was thus finally determined.” After the three-month labor service, the studies began in the winter semester 1936/37 in the course “applied mathematics” at the Technical University Berlin-Charlottenburg. The study plan provided for attending the lecture “Experimental Physics” from the first semester on. The lecture was held enthusiastically by Prof. Hans Geiger, which led Liselott Herforth to consider changing her field of study. As a precaution, she took physical chemistry, electrical engineering and mechanical engineering. This enabled her to stay on schedule if she changed to the physics program. She still took the intermediate diploma in mathematics in 1938. After the examination, she changed to physics for her main course of study. She was hired directly by Prof Geiger as an assistant. She was also given a diploma topic by him: “Determination of the half-life of ThC’ with the aid of a coincidence amplifier with continuously variable resolving power”. She built the coincidence amplifier for this herself and graduated with a diploma in 1940.
However, the diploma thesis needed a revision by Prof. Geiger: “Read the papers in the Zeitschrift für Physik, only the essentials, only the most necessary, write the thesis again.” After this was done, she worked for two more years until January 1943 at the Technical College in the departments of mathematics and physics. Her research dealt with counting tubes, cosmic rays, the already familiar coincidence amplifiers, and radioactive radiation. Radioactive radiation would later be the main subject of her scientific career. She made the dissertation “Scattering of mesons in lead measured in counting tubes”. She did not pass the oral examination for the dissertation. Her boss at the time, Werner Heisenberg, then said, “Don’t worry about it.” Mediated by Heisenberg, she went to the Physics Institute of the University of Leipzig on June 1, 1943. On March 1, 1944, she moved to the University of Freiburg, where she was an assistant to the director of the Physics Institute. Two years later on April 1, the position of industrial physicist followed at the Oberspreewerk. Despite a salary increase after only half a year, she left the plant at the end of December to pursue her doctorate.

From January 1, 1947, to January 31, 1949, she completed her doctorate at the Kaiser Wilhelm Institute for Physical Chemistry and Electrochemistry in Berlin Dahlem on the topic “Fluorescence of Organic Substances on Excitation with Alpha, Beta, Gamma Radiation” posed by Hartmut Kallmann. The doctorate was to be carried out without payment.
After four months, Ms. Herforth already had small results to show and was thus able to receive a scholarship. As the amount of successful data grew, she was hired as a research assistant and received a salary. Her doctoral supervisor Hartmut Kallmann gave a lecture based on her data on the excitation of fluorescence of organic liquids as early as 1947. The world’s very first publication on this topic was published in 1949 in the Annalen der Physik jointly authored by Liselott Herforth and Hartmut Kallmann. Parallel to the research on her dissertation, she worked on experiments which were started in Leipzig and from this her first publication followed already in 1947: “Measurement of very low ionizations with the flow ionization chamber according to G. Hoffmann”. Liselott Herforth had set herself a time limit of 2 years due to the situation of doing an unpaid doctoral thesis. She was able to fall short of this by 3 months, so that the doctoral thesis was submitted on September 13, 1948. The disputation took place on November 1, 1948. The examination was passed successfully and the overall grade of the doctoral thesis was “Good”, with the written part being rated “Very Good”. She applied to the Technical University for a scheduled assistant position and for an assistant position in Berlin Buch. Later, in an interview, she recalled the trip to the interview as follows: “It is 35 years ago today, almost to the day, when I took the S-Bahn from West Berlin, along the rain-soaked Lindenberger Weg, to introduce myself to Walter Friedrich shortly after my doctorate at the Technical University of Charlottenburg, in the hope of finding employment in the newly founded Academy Institute for Medicine and Biology headed by him, and especially in his department of biophysics, which was still being set up. I was not entirely comfortable with this, for I had no idea of medicine, biology, or biophysics […].”
She would have received both positions, but due to administrative delays she withdrew her application to the TU Berlin-Charlottenburg already on December 1 and started her new job in Berlin-Buch on February 1, 1949 in the Department of Biophysics as a scientific assistant. On that day, only a final positive decision was made about her application to the TU Berlin-Charlottenburg. She later said of the move to East Berlin, “It was the best decision in my life.” In the meantime, there were fewer career opportunities for female scientists in the sectors of the Western Allies than had been the case at the time of the war due to the “shortage of men.”
Looking back on her scientific position in Berlin-Buch, Ms. Herforth says of the early days: “I could not imagine at all what X-ray structural analysis could have to do with the institute’s task, which was to study cancer. Secretly, however, I cherished the hope that what I had learned and mastered so far, namely the Geiger-Müller counting tube technique and scintillation counting, would also be needed here one day, and perhaps also my experience with fluorescent substances. But these were only faint hopes, of which I did not dare to speak when I came face to face with Walter Friedrich.” In Berlin-Buch, she first had to learn the ropes from Käthe Dornfelder, who dominated Walter Friedrich’s laboratory in many scientific areas. Liselott Herforth’s task was to “create the experimental preconditions for the preliminary investigations for the development of an apparatus for the light-photographic determination of the intensities of X-ray reflections.”

Gradually, she was to wean herself off Mrs. Dornfelder and was able to design her “Labor Herforth” in two rooms according to her scientific requirements as early as 1950.
Since 1949 she had held a habilitation aspirancy at Humboldt University and in May 1953 she was asked by the dean of the Faculty of Mathematics and Natural Sciences to submit the documents required for the habilitation. Since she had numerous publications to show, the writing and submission of a habilitation thesis would be waived. After receiving the letter, Ms. Herforth inquired at the University of Leipzig whether habilitation would be possible and desired there. The habilitation in Leipzig was also possible without the additional writing of a habilitation thesis and so she habilitated at the University of Leipzig in the same year. For her habilitation in Leipzig she decided to follow her father’s maxim: “Always go where you are needed most.” Not only in this but in all important career decisions she followed this maxim. In addition, she saw it as a purposeful detachment from her previous laboratory “Dornfelder”. The University of Leipzig applied for her to become a lecturer in radiation physics as of September 1, 1954, and so she taught courses and began her research activities here in the fall.
As her career progressed, Ms. Herforth quickly learned what it meant to lead a team and guide it to the best possible performance. When decisions had to be made, she did not act hastily or without thinking. Often, she had said, “I’ll sleep on it for a night, then decide.”

In addition to her work at the University of Leipzig, she remained the fully responsible head of the laboratory in Berlin-Buch, with an attendance requirement of two days a week. At the University of Leipzig, however, she worked full-time for only one year.
On September 1, 1955, she began her employment at the institute of Carl Friedrich Weiss, whose official name would be “Institute for Applied Radioactivity” (IaR) and which was founded under the umbrella of the “Institute for Organic Chemical Industry”. For this activity, her employment contract with the University of Leipzig was replaced by an individual contract with the Ministry of Heavy Industry and an additional contract with the University of Leipzig. For she continued to give lectures here. The Institute for Applied Radioactivity was still under construction and was opened on January 1, 1956. She was the head of the training department, which had to be built up and shaped from scratch. Priority was given to practical training for employees in industry, universities and medical institutions, in which they were trained to work with radioactive substances. The scripts and work instructions for the practical course resulted in the internationally recognized practical course book “Praktikum der Radioaktivität und der Radiochemie”.
The Faculty of Materials Management of the Technical University in Leuna-Merseburg, founded in 1954, applied for the appointment of Mrs. Herforth as professor with the task of teaching the subject “Applied Radiochemistry” as of September 1, 1957. The expert reports on Mrs. Herforth requested for this purpose stated, among other things: “Mrs. Herforth, however, has not only become a cleanly working and experienced experimental physicist, but also at the same time a pedagogically capable teacher. Her lectures are exemplary and exquisitely prepared. [ ] Actually, she is constantly overworked because she knows no sparing against herself.” Liselott Herforth gave all lectures on metrology, radioactivity, dosimetry and radiation protection in Merseburg. She did not receive her teaching license until early December, but then retroactively for November 1. She also remained the head of the teaching department at the IaR in Leipzig and continued to give her lectures on artificial radioactive isotopes at the University of Leipzig.

When the Atomic Energy Conference was held in Geneva in 1958, Liselott Herforth was one of the 35 scientists from the GDR (German Democratic Rebublic) who participated as observers. On September 1, 1960, she became a professor with a full teaching assignment for applied radioactivity at the Nuclear Technology Faculty of the Dresden Technical University (TH).
This also included the research activities associated with her subject. The beginning in Dresden, however, was fraught with uncertainties. She remembers it later as follows: “Hardly had I arrived when the question of dissolving Faculty K (Faculty of Nuclear Engineering at the TH/TU Dresden) was discussed in all committees, even outside the TH. Since I still had little insight into the overall university events at the TH and still had little contact with colleagues, I initially sat in Zelleschen Weg 19 still rather isolated and still had enough time to think at that time. At first, I saw my task in acquiring the trust of all employees in the institute […], because I was to take over this institute one day. Acquiring trust also happened very quickly, because the employees were very happy that someone was there who had time for them, who supervised doctoral theses, who worked exclusively at the TH. That with all the time to think didn’t take long either, then I was involved in the faculty work.”

Her father also gave her the life advice: “Always go where you are needed most!” which she based her career on. In April 1962, she decided to join the SED. At a time when she had already reached the top of her scientific career. In her application for admission to the SED, she justified her wish as follows: “I am convinced that as a party member I will be able to fulfill my tasks as a university teacher, namely to train and educate the most expensive asset of the state, its youth, even better than before.”
On the subject of women’s equality, many quotes from her have come down to us:
“If a girl can dance or sing well, parents consider whether the daughter could go to ballet school or music school. They know: If you don’t start practicing early, nothing will happen. However, if a daughter performs very well in math class, what parent is going to focus attention on that and make it clear to the child that there are […] professions for which math provides good prerequisites!”
“All work for the development and advancement of women must become the principle of managerial activity in all institutes, training centers, and industries.”
“Why are there too few women in leading positions in industry? We must also ensure with girls already at university that, in addition to acquiring technical knowledge, they are also educated to become personalities who already know what leadership actually means.”
Academic careers were more difficult for women then, as they are now. In a talk at a women’s congress in 1964, she posed the question, “After all, what does the practice look like?”
Her answer and assessment immediately followed, “The path from student to university lecturer is a long one, and not only is it long, but this is also a profession in which, especially in the technical field, there is no educational qualification. First of all, the woman has to work very intensively, especially in the years when she starts a family and the children are small, that is, between the ages of 20 and 30. She has to get her diploma, get her doctorate. But that’s not the end of it. Then comes the habilitation.
That takes another four years or so. […] So it’s particularly difficult for a woman to achieve that goal if she doesn’t want to give up having a family.”
Her suggestion for increased promotion of women followed: “Individual promotion plans, taking into account family and other conditions, are to be worked out for female junior scientists. They should be provided with continuous and systematic academic support by professors and institute staff. The professors and institute collectives must provide them with continuous and systematic academic support.”
On October 29, 1965, Ms. Herforth was appointed the first German female rector. Ms. Herforth classified her election as rector as a sign of equal rights:

“This proves once again that all avenues are open to our women in the GDR to take on the offices associated with the highest responsibility. Especially at a technical university, this should help us to attract more women and girls to technical studies.” She was elected for two years and re-elected for another year (until 1968).
The day of her appointment coincided with the matriculation of the new degree program. In her speech she had much to say to the new students: “When you have finished your studies after five years, a job is waiting for you in industry or in an institute or at a university. During these five years you will have only one concern, and that will and must be to develop into a socialist personality, equipped with the best professional knowledge. This concern is also our concern, i.e. the concern of the entire teaching staff. […] We will help you to get started by providing particularly intensive instruction in the first semesters. […] The demands on you will grow. However, you will soon realize that if you make good use of the 24 hours of the day and manage your work properly, you will still have time for sports, music, theater and other cultural and social activities.”
In her speech, she also addressed the word directly to all female students. She indicated what was possible and what was expected in return. What each one had to achieve on her career path: “You are living in a good and happy time for us women; all professions are open to us. But, and this is what I would like to leave you with today, we have to prove anew every day that we have rightly earned our place in society. So, when you start your studies today, many fellow students and university teachers
will look to you with great expectations. […] All opportunities will be given to you to hold the finest and highest offices, but you will have to work out the prerequisites for this yourself. We can help you in this, but the creation of the professional prerequisites as well as the development into a socialist personality will come to you in the same way as to the male students.”
In addition to her appointment as rector, she also became a State Council member in 1965. In a report to the State Council we find her assessment of this: “The combination of being a rector and at the same time a member of the State Council and vice versa, being a rector as a member of the State Council, is a very good combination especially for me as the first female rector, which supports my work very much and makes many things easier for me. Of course, not all rectors can be members of the State Council, but it is important that the rector takes a clear and unambiguous position on any issue […] that may arise.”
“It is impossible to endorse the view, sometimes held at the university, that studying is a selection process and that the task is to identify alleged failures, if possible at the beginning of the study period, and to exmatriculate them in time. It is the duty of every member of the university to enable all students admitted to study to achieve the study goal.”
From 1969, she was a professor in the Physics Section. In that year she also received the distinction of becoming a “full member of the Academy of Sciences.” In an expert opinion on this, it is said: “Ms. Herforth has a good eye for technical realizations of scientific results and has very good relations with
industry,” and “It is astonishing that Ms. Herforth was able to carry out her scientific and organizational activities alongside very stressful political work.”
What is still true today, Ms. Herforth communicated even then: “We should keep in mind that a large part of our research capacity is carried by our students. If this were not so, we would not be able to fulfill our research obligations at all. At the same time, a considerable amount of effort has to be expended by university teachers and research assistants in order to achieve high levels of performance. In the process, the supervising staff can do less direct research themselves.”
In 1974, at intervals of one year each, she received an honorary doctorate from the Technical University of Chemistry in Veszprém (Hungary), the “Medal of Merit of the National People’s Army” and in 1976 the Humboldt Medal in gold.
Women retired at the age of 60 at that time. Men, on the other hand, did not go until they were 65. At the age of 60, in 1976, Prof. Herforth felt intellectually capable and physically up to her tasks. It has been said that she felt this was an injustice and would have liked to continue to teach at the university to her full extent. Then, in mid-August, an agreement was reached with the University of Dresden that she could continue to work for the university as of September 1, 1977. Her duties were to revise the internship book in addition to chairing disputations and writing dissertation reviews.
At age 65, Mrs. Herforth most likely still expected to be able to run one more time for the Volkskammer, of which she had been a member since 1963. However, she was informed in 1981 that she would not be nominated again.
Around the time of her 75th birthday in 1991, she moved into a retirement home. She continued to be active in science academies. However, she did not finish the memoir she had begun.
You can download this article in German here:
Literature:
- Voss, Waltraud: Liselott Herforth; Die erste Rektorin einer deutschen Universität, transcript Verlag, 2016, ebook.
- https://tu-dresden.de/ua/archiv-bestaende/medienarchiv/professorenbildnisse
- https://www.bbaw.de/die-akademie/akademie-historische-aspekte/mitglieder-historisch/historisches-mitglied-lieselott-herforth-1109
If you find any errors or if you are the copyright holder of an image and do not agree to its use on this site, please contact beam@chemie.uni-halle.de .

Jocelyn Bell Burnell
*1943
“If physics interests you, do it! Study it. It’s huge fun, it’s fascinating, exciting and a great profession!”
Jocelyn Bell was born in Lurgan in Northern Ireland on July 15, 1943. Her father was an architect and built the planetarium for County Armagh where they lived. As a little girl she often visited this planetarium and was encouraged by the staff working there to become involved in astronomy. Later she found a book on astronomy by Fred Hoyle on her father’s bookshelf. At the elementary school, Lurgan College, it came about that the boys received science lessons and the girls received home economics lessons during that time. When she told her parents this, three girls and all boys sat in the following science class.
Read more…
When she failed to graduate from college in 1956, her parents sent her to the “Mount School” in York. Here she was inspired by her physics teacher who told her, “you don’t have to learn tons of facts. Just learn a few key facts and then apply them and develop everything else from there”. This teacher showed her how simple physics really is. After graduating from school, she began studying physics in Glasgow. At the beginning she had difficulties because of her low knowledge of science at school. But she studied determinedly and graduated with a bachelor’s degree in physics in 1965. She then went to Cambridge to join the research group of Anthony Hewish, where she wanted to do her PhD in radio astronomy.

For the first two years, she and half a dozen people were busy building a radio telescope. Her presence as a female Ph.D. student was special; the other women were all secretaries.
If she had not known full well that she wanted to be a radio astronomer, she probably would not have been able to endure either graduate or doctoral study in the misogynistic environment. In her time, there were no female role models for the profession of astronomer.

The first data from the radio telescope could be received in 1967. Jocelyn Bell noticed signals that her thesis advisor dismissed as interference or gave as the reason that the telescope had been set up incorrectly by her.
The telescope worked perfectly however. Also, the measurable signal came regularly even after all possible interfering signals had been turned off. Her signal still let measure reliably. She had found a fast rotating neutron star. However, she also realized that presenting only one neutron star was not enough convincing enough. So she went through all her measurement records again. These were meters of paper, which had to be sifted. In fact, she found another neutron star that was emitting signals as it rotated. The press later shortened these “pulsating radio stars” to “pulsars,” by which they are still known today. They are remnants of large stars that explode at the end of their lives. The tiny star spins and the signal is measurable if the telescope is properly aligned. Her data were published in Nature in 1968, the same year she defended her doctoral thesis and married.
The marriage gave her the middle surname Burnell. When she proudly showed her wedding ring to her colleagues, she received criticism instead of congratulations. At the time, it was not proper for a married woman to go to work because it implied that her husband did not earn enough to support the family. She taught at Southampton University from 1970 – 73. In 1973 her son was born, initially she worked part-time after that. From 1974 to 1982 she was a professor at University College London. In 1974, the Nobel Prize was awarded to her thesis advisor, Anthony Hewish, and the then director of the institute, Martin Ryle, for their discovery of pulsars. The scientific public found that Jocelyn Bell Burnell was passed over for the prize. She herself said that it probably played a role that she was still a PhD student at the time, but that being a woman probably also played a not insignificant role. Later, in such a case, the doctoral students were awarded together with their supervisors. Due to the constant change of her husband’s place of work, she also moved regularly. As a result, she was not able to conduct research on one topic at a time, but rather this changed increasingly. She found this very frustrating. After her son was born, she started working part-time, and also taught at the Open University from 1973 to 1987. In 1986 she became head of the department that looked after the James Clerk Maxwell telescope in Hawaii. She worked at the Royal Observatory in Edinburgh from 1982 to 1991. In 1991 she became Professor of Physics at the Open University in Milton Keynes. In 1993, the Burnell couple divorced.

From 2001 – 2004 she was Dean of the Faculty of Science at the University of Bath. From 2004 she was a visiting professor at Oxford. Many interview videos of her can be found on the internet, she gave a TEDtalk and did science communication before science communication even existed.
She sees herself in the important role of a female role model in science. Because she herself didn’t have those role models, and if she hadn’t known exactly that she wanted to be a radio astronomer, the vagaries and also injustices might have kept her from that career. In 2007, she was ennobled by the Queen and has been “Dame Jocelyn Bell Burnell” ever since.
You can download this article in German here:
Literature:
- https://www.uni-muenster.de/Physik/department/equality/women_and_physics/history/jocelyn_bell_burnell.html
- https://www.britannica.com/biography/Jocelyn-Bell-Burnell
- https://www.biography.com/scientist/jocelyn-bell-burnell
- https://www.fh-kiel.de/campus/berichte-vom-campus-fh-kiel-news/campusleben-fh-kiel-news/2007-2011/jocelyn-bell-burnell-die-entdeckerin-der-pulsare/
- https://physik.cosmos-indirekt.de/Physik-Schule/Jocelyn_Bell_Burnell
If you find any errors or if you are the copyright holder of an image and do not agree to its use on this site, please contact beam@chemie.uni-halle.de .